A team of scientists at the RIKEN Centre for Sustainable Resource Science (CSRS) in Japan headed by Ryuhei Nakamura has discovered a new practical and sustainable approach for generating hydrogen from water. On the contrary to current approaches, the new method does not need rare metals that are short in supply or costly.
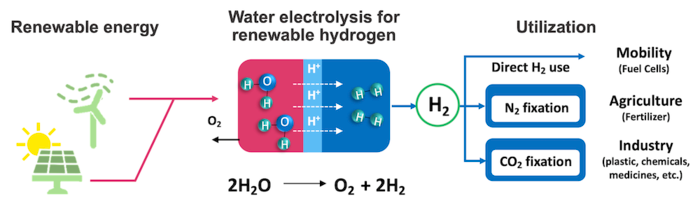 This schematic shows the concept for sustainable hydrogen production. Electricity from renewable sources (solar, wind) is used to split water into oxygen and hydrogen (electrolysis). The hydrogen can then be used as fuel, to help make fertilizer from ammonia, and in other industries. Image Credit: Riken.
This schematic shows the concept for sustainable hydrogen production. Electricity from renewable sources (solar, wind) is used to split water into oxygen and hydrogen (electrolysis). The hydrogen can then be used as fuel, to help make fertilizer from ammonia, and in other industries. Image Credit: Riken.
Instead, hydrogen for agricultural fertilizers and fuel cells can be now produced using manganese and cobalt, two equally common metals. The research was published in Nature Catalysis.
Hydrogen is a clean fuel that produces water as its only by-product, unlike traditional fossil fuels that produce carbon dioxide on combustion. The energy grid can be made renewable, clean and sustainable if hydrogen can be retrieved from water with the use of renewable electricity.
In addition, hydrogen is the vital ingredient required in the production of ammonia that is employed in almost all synthetic fertilizers. However, instead of extracting hydrogen cleanly from water, ammonia plants are now utilizing fossil fuels to generate the hydrogen they require.
The unsustainable and expensive nature of electrolysis — the hydrogen extraction process — could be one reason.
This is primarily due to a lack of good catalysts. In addition to being able to withstand the harsh acidic environment, the catalyst must be very active. If not, the amount of electricity needed for the reaction to produce a given amount of hydrogen soars, and with it, so does the cost.
Ryuhei Nakamura, Study Lead, Centre for Sustainable Resource Science, RIKEN
At present, rare metals like platinum and iridium are the most active catalysts for water electrolysis, creating a dilemma, as they are costly and regarded as “endangered species” among metals.
Right now, iridium production of about 800 years’ worth — an amount which might be impossible to exist — would be required for switching the earth to hydrogen fuel. On the contrary, abundant metals, like iron and nickel, are not active enough and dissolve immediately in the environment of harsh acidic electrolysis.
The scientists considered mixed cobalt and manganese oxides in their quest for a better catalyst. Cobalt oxides could be active for the required reaction but corrode very rapidly in the acidic environment. Manganese oxides are more stable in comparison but are not that active.
By mixing them, the scientists expected to benefit from their complementary properties. The scientists also considered the high current density requirement for practical application outside the lab environment.
For industrial scale hydrogen production, we needed to set our study’s target current density to about 10 to 100 times higher than what has been used in past experiments. The high currents led to a number of problems such as physical decomposition of the catalyst.
Shuang Kong, Co-First Author, Centre for Sustainable Resource Science, RIKEN
Consequently, they overcame these problems, through trial and error, and found a stable and active catalyst by introducing manganese into the spinel lattice of Co3O4, creating the mixed cobalt manganese oxide Co2MnO4.
Upon assessment, it was revealed that Co2MnO4 functions very well. Activation levels were near to those of advanced iridium oxides. In addition, the new catalyst remained for more than two months at a current density of 200 milliamperes per square centimeter, which could make it efficient for practical use.
The new electrocatalyst could be a game-changer compared with other non-rare metal catalysts that can only usually last a few days or weeks at much lower current densities.
We have achieved what has eluded scientists for decades. Hydrogen production using a highly active and stable catalyst made from abundant metals. In the long run, we believe that this is a huge step towards creating a sustainable hydrogen economy. Like other renewable technologies such as solar cells and wind power, we expect the cost of green hydrogen technology to plummet in the near future as more advances are made.
Ailong Li, Study Co-First Author, Centre for Sustainable Resource Science, RIKEN
Finding ways to expand the lifetime of the new catalyst and enhance its activity levels further is the next step in lab. “There is always room for improvement,” concludes Nakamura, “and we continue to strive for a non-rare metal catalyst that matches the performance of current iridium and platinum catalysts.”
Journal Reference:
Li, A., et al. (2022) Enhancing the stability of cobalt spinel oxide towards sustainable oxygen evolution in acid. Nature Catalysis. doi.org/10.1038/s41929-021-00732-9.