In a recent study published in the open-access journal Fermentation, researchers from Italy analyzed the extraction of chitin and chitosan from fungal sources rather than shellfish wastes.
 Study: Chitosan Production by Fungi: Current State of Knowledge, Future Opportunities and Constraints. Image Credit: grebeshkovmaxim/Shutterstock.com
Study: Chitosan Production by Fungi: Current State of Knowledge, Future Opportunities and Constraints. Image Credit: grebeshkovmaxim/Shutterstock.com
This study focused on various production processes, parameters affecting the production processes, advantages and limitations in comparison with the shellfish-based extraction process, and the prospect of promoting a circular economy.
What are Chitin and Chitosan?
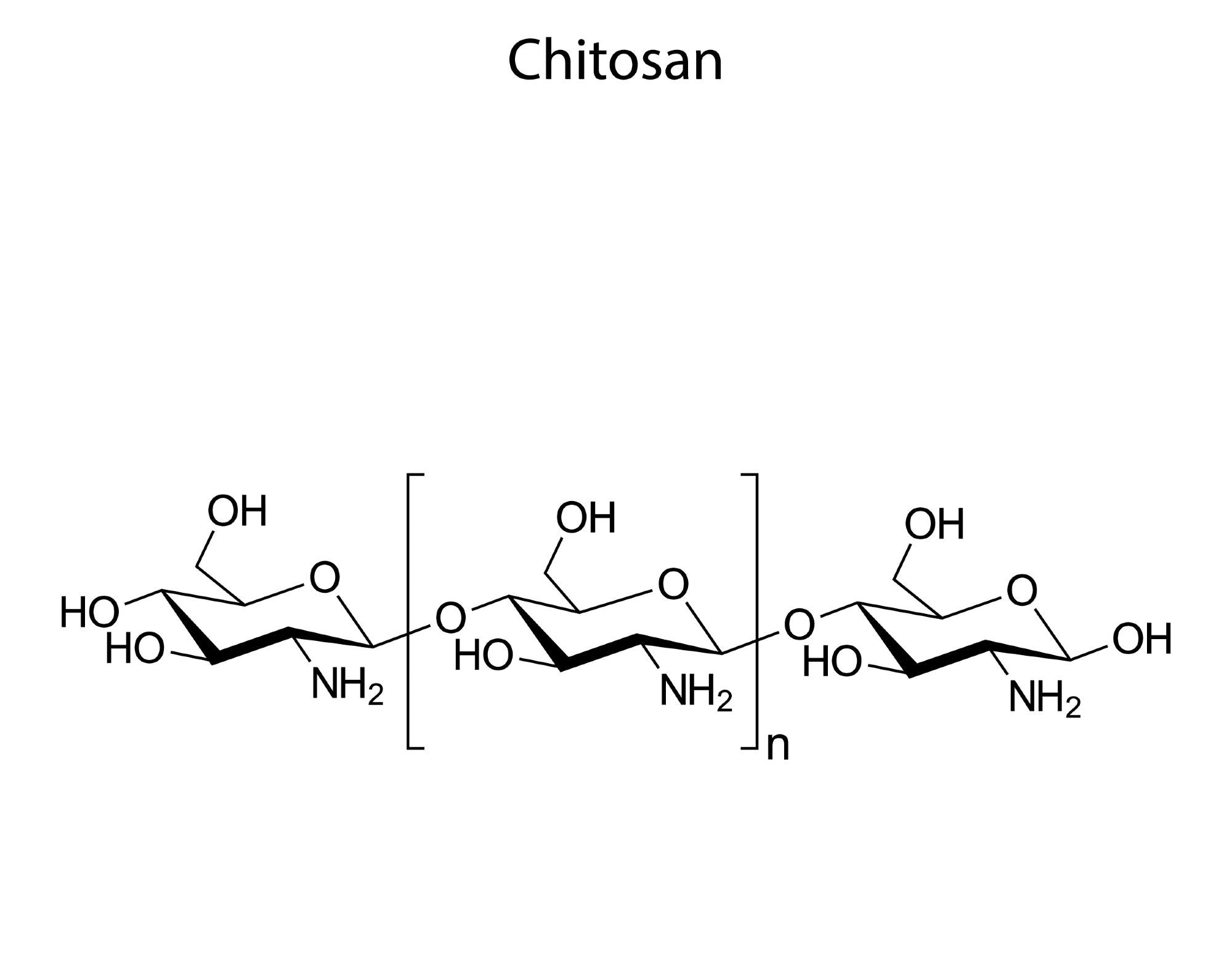
Both chitin and chitosan are polysaccharides. Chitin is the second most abundant biopolymer present on earth after cellulose, which is an amide derivative of glucose consisting of N-acetyl-D-glucosamine (GlcNAc). Meanwhile, chitosan is a linear polysaccharide with free amine groups, which is primarily made of β‐(1‐4)‐linked D‐glucosamine (GlcN) copolymer.
Chitosan is often derived from chitin through deacetylation. Both of them are widely used in biomedical applications owing to their biocompatibility, nontoxicity, biodegradability, and ability to form thin films. As per an estimate, the global demand for chitin and chitosan will grow 14.8% compounded annually between 2020 to 2027.
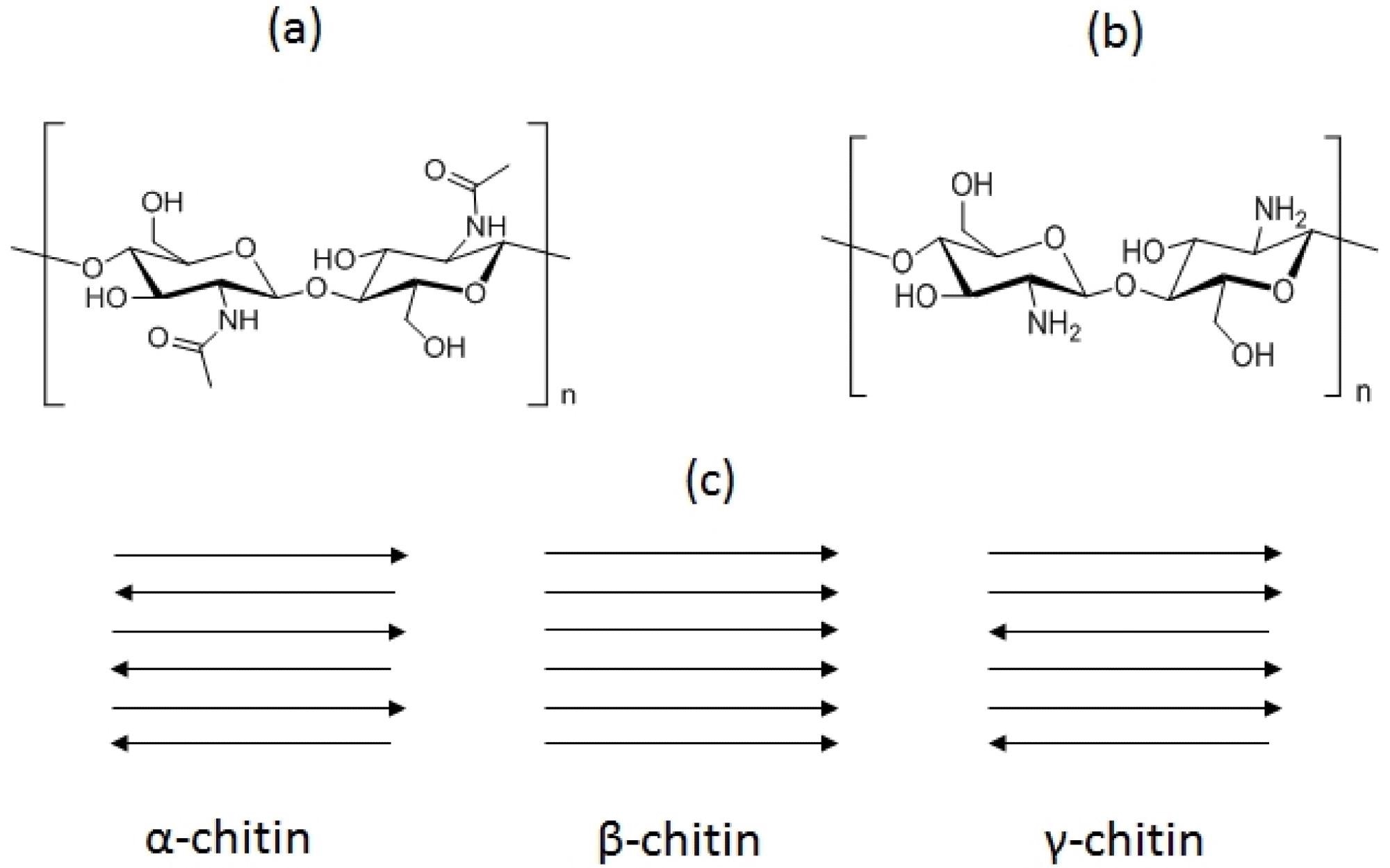
Basic structures of chitin (a) and chitosan (b) and chitin allomorphs (c). The tips of the arrows indicate the positions of the reducing ends of the chains. Image Credit: Crognale, S et al., Fermentation
Chitin is found in nature in three crystalline phases namely, α, β, and γ. Among these, α-chitin is the most readily available one, which is found in arthropods and fungi. β‐chitin is present in cephalopods, whereas γ-chitin is the rarest one. The dry weight of crab and shrimp processing wastes contain 14% to 27% chitin, respectively.
Traditional Extraction Process of Chitin from Crustacean Shell Waste
The traditional extraction and deacetylation process of chitin involves three major steps. In the first step, strong acids such as hydrochloric acid (HCl) with a concentration of 0.6-1.1 M are used to demineralize the biowastes at room temperature. In the second step, alkaline sodium hydroxide (NaOH) with a concentration of 0.12-5.0 M is used to deproteinize at a temperature up to 160 °C.
Finally, the pigments such as astaxanthin and β‐carotene are removed using acetone. All these biomaterial unfriendly steps along with the variable composition of raw materials make unsteady physicochemical properties of chitosan vary from batch to batch.
Advantages of Chitin Extraction from Fungi
First of all, the fungal sources of chitin can be cultured in large quantities for commercial purposes, but such large-scale cultivation is difficult for crustacean shells. Moreover, they are non-seasonal and reliable sources with consistent properties and raw material composition. Fungal chitin also contains a lower amount of ash compared to shell-extracted chitin.
Most importantly, fungal chitin does not require the acid-based demineralization or decolorization step. It can also promote a circular economy by profiting mushroom growers and other biotechnological industries. Additionally, the fungal chitin extraction process is more environment-friendly, can be obtained over a wider range of molecular weight compared to shell-extracted chitin, does not contain allergenic proteins such as tropomyosin, and the disposal cost of fungal-based waste is much lower.
Fungi that Produce Chitin and Chitosan
Three main classes of fungi Ascomycetes, Basidiomycetes, and Deuteromycetes contain a large amount of chitin in their cell walls. Chitosan is somewhat less found in the cell walls of fungi, but Zygomycetes class fungi contain the highest amount of chitosan. In the Zygomycetes class, Mucorales order fungi belonging to Cunninghamellaceae, and Mucoraceae families are major sources of fungal chitosan.
Some common examples of them are Absidia (A.) blakesleeana, A. coerulea, Cunninghamella (C.) bertholletiae, bertholletiae, C. echinulate, Gongronella butleri, Mucor (M.) indicus, M. rouxii, Rhizomucor (R.) miehei, Rhizopus oryzae and Syncephalastrum (S.) racemosum. Some fungi belonging to the Absidia and Rhizopus genus of the Zygomycetes class are pathogens to animals or humans.
They cause mucormycosis in humans and zygomycosis in bovines. Colletotrichum lindemuthianum is a causative agent of ‘anthracnose’, which is a plant disease that affects several temperate and tropical climates crops. Thus, handling such fungi requires utmost care.
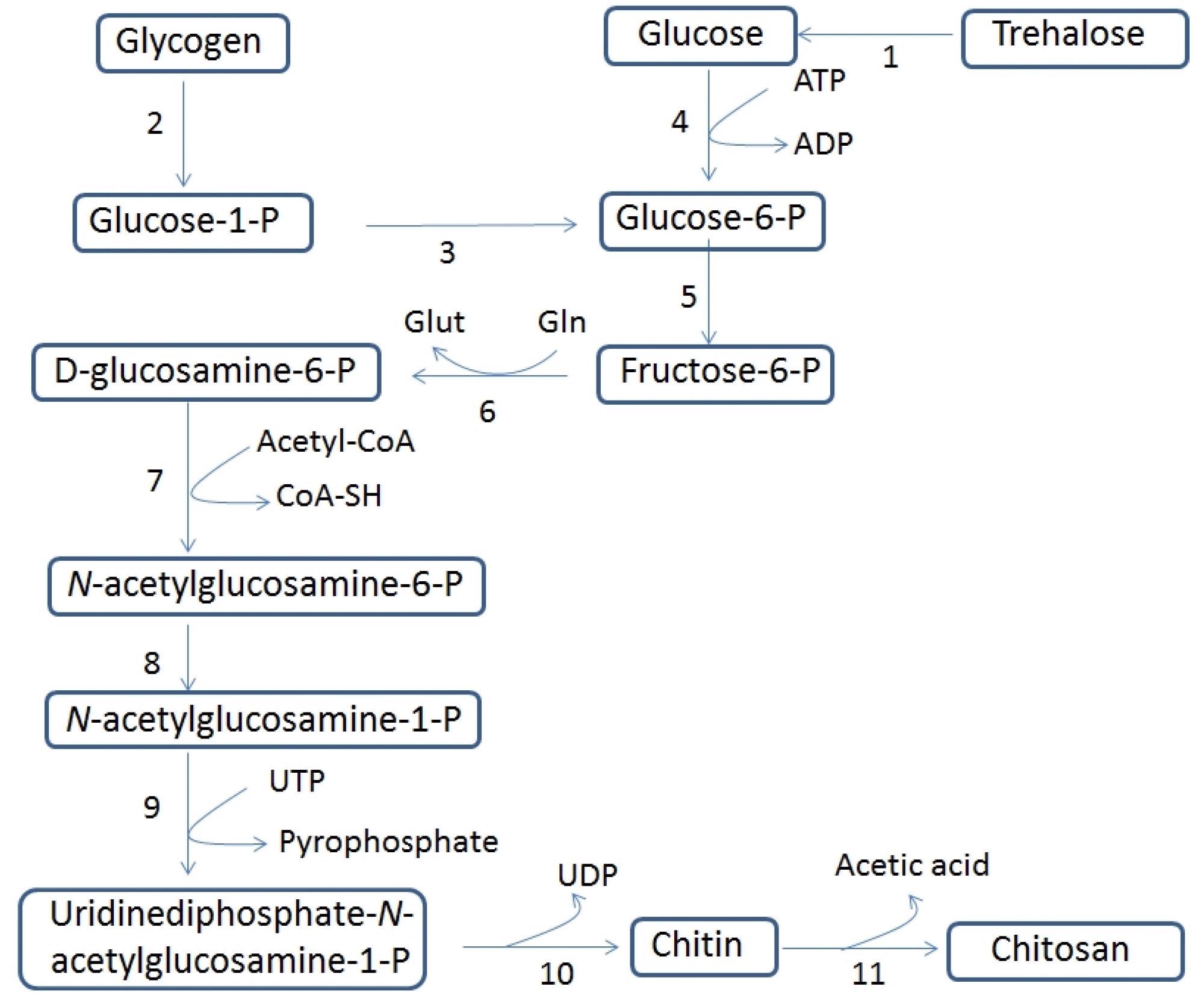
Biosynthetic pathway of chitin and chitosan. The following numbering has been assigned to the enzymes that catalyze each reaction: 1, trehalase; 2, glycogen phosphorylase; 3, phosphoglucomutase; 4, hexokinase; 5, glucose-6-phosphate isomerase; 6, glutamine-fructose-6-phosphate amidotransferase; 7, glucosamine-6-phosphate N-acetyltransferase; 8, N-acetylglucosamine-phosphate mutase; 9, UDP-N-acetylglucosamine pyrophosphorylase; 10, chitin synthase and 11, chitin deacetylase. The abbreviations used are as follows: acetyl CoA, acetyl coenzyme A; ADP, adenosine diphosphate; CoASH, coenzyme A; Gln, L-glutamine; Glu, L-glutamate; UDP, uridine diphosphate; UTP, uridine triphosphate. Image Credit: Crognale, S et al., Fermentation
Production Processes
Solid-state fungal (SSF) chitosan is extracted from fungi that are cultured on solid substrates such as rice and wheat straw, hardwood sawdust, cottonseed hulls, potato chip processing waste, sweet potato pieces, and soybean residues. A. niger and M. rouxii fungi grown on soy-derived solid substrates achieve peak concentration in 12 days with an average volumetric productivity of 59 and 119 mg kg−1 h−1, respectively.
Another group of fungi is cultured in a submerged state inside specific biofluids such as potato dextrose broth (PDB), yeast extract‐malt extract medium (YM), yeast extract‐peptone‐dextrose (YPD) medium, glucose‐peptone‐yeast extract (GPY), and glucose peptone.
Conclusions
To conclude, this study focused on fungal-based chitin and chitosan production. Fungal chitin and chitosan offer several advantages over crustacean shell-extracted chitin and chitosan. Moreover, both SSF and liquid submerged processes utilize readily available biowastes, which is good from an environmental perspective.
However, some pathogenic fungi need careful handling otherwise they may harm crops, animals, and even humans. Fungal chitin production requires a more competitive approach to reduce the cost of production compared to conventional processes.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.
Source:
Crognale, S., Russo, C., Petruccioli, M., D’Annibale, A., Chitosan Production by Fungi: Current State of Knowledge, Future Opportunities and Constraints. Fermentation 2022, 8, 76. https://www.mdpi.com/2311-5637/8/2/76