In a recent study published in the open-access journal Nature Communication, researchers developed a novel non-thermal plasma-assisted rapid hydrogenolysis method to recycle plastic polystyrene (PS) waste to produce ethylene and hydrocarbon gases.
Study: Non-thermal plasma-assisted rapid hydrogenolysis of polystyrene to high yield ethylene. Image Credit: Irina Anosova/Shutterstock.com
This method demonstrated an excellent high yield of more than 40 wt% and ethylene selectivity of more than 70%. The highly active hydrogen plasma effectively broke bonds in PS and promoted rapid hydrogenolysis at ambient temperature and atmospheric pressure. This method is ideal for the extraction of light-weight hydrocarbons (i.e., C1-C3) from plastic wastes and the recycling of complex plastic wastes.
Background
Despite a public motion to bat for sustainable, eco-friendly products, the use of plastic is consistently growing. Industry 4.0 has not found a suitable alternative to plastic products owing to its high strength to weight ratio, durability, and low manufacturing cost. In the year 2018, the total production of plastics reached 400 million tons. As a result, the quantity of plastic wastes (PWs) is also increasing. Out of these, only less than one-fifth of PWs are recycled properly; meanwhile, the rest end up in landfills, incineration, or get into the environment directly.
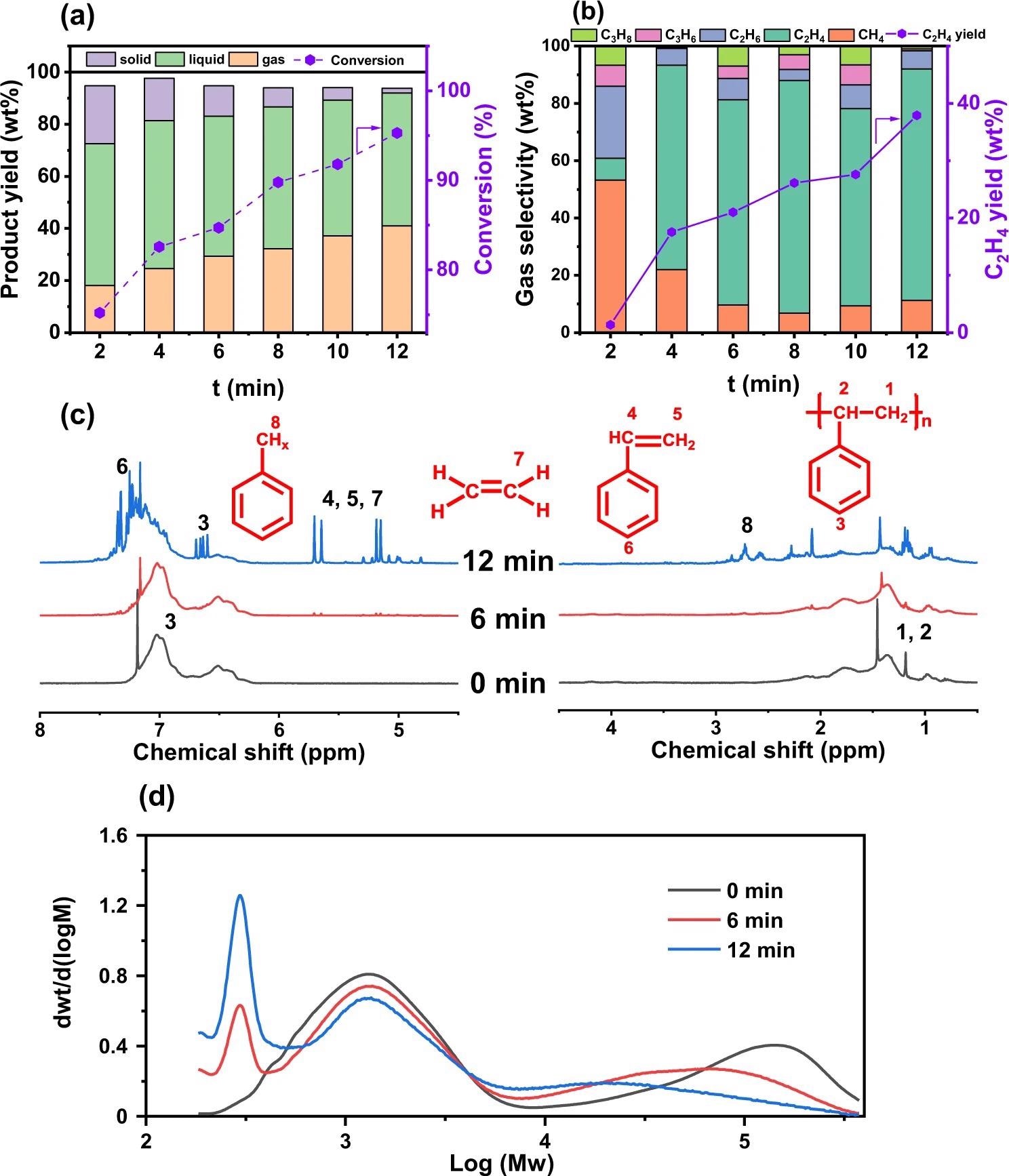
Time-dependent reaction properties of plasma-assisted PS hydrogenolysis. a Product yield (Y) and conversion (X) and (b) gas product selectivity and ethylene yield as a function of reaction time (t). c 1H NMR spectra and (d) GPC spectra of liquid products after different reaction time (PS-0min, PS-6 min, and PS-12 min). Reaction conditions: νH2 = 100 ml/min, PH2 = 101 kPa, P = 90 W. Image Credit: Yao, L et al., Nature Communications
Furthermore, the recycling of low-grade plastics is primarily limited to small-scale remolding, in which only thermoplastic PWs are shredded, heated, and remolded. This process is not that effective for thermosetting PWs. Meanwhile, the chemical recycling processes include pyrolysis and gasification.
The resulting products of chemical recycling are less value-added synthesis gases and carbon-based materials. However, both thermal remolding and chemical disintegration processes require very high operating temperatures in a range of 700-1300 K, which makes them cost-inefficient.
Plastic hydrogenolysis is an emerging technology in recycling PWs, which requires relatively less temperature in a range of 600-700 K, but the required pressure is still high in a range of 10-30 bar, and the reaction time is more than 6 hours.
About the Study
In this study, researchers used a non-thermal hydrogen (H2) plasma for rapid hydrogenolysis of pure and used PS under ambient temperature and atmospheric pressure. The plasma provided a conducive medium for the generation of H2 species in the form of ions and radicals, which broke the C-C bonds of the polymeric chain of PS.
The plasma-assisted hydrogenolysis reaction was performed in a quartz tube consisting of a dielectric barrier discharge (DBD) plasma generator. The produced H2 plasma has a temperature equal to the ambient temperature, and the volume of the plasma zone was ~6.28 cm3. In each run, 200 mg of PS pellets of size 125-250 µm were packed in the DBD reactor. The plasma had a power of 90 W, an H2 flow rate of 20-100 ml/min, and a reaction time of 2-12 min.
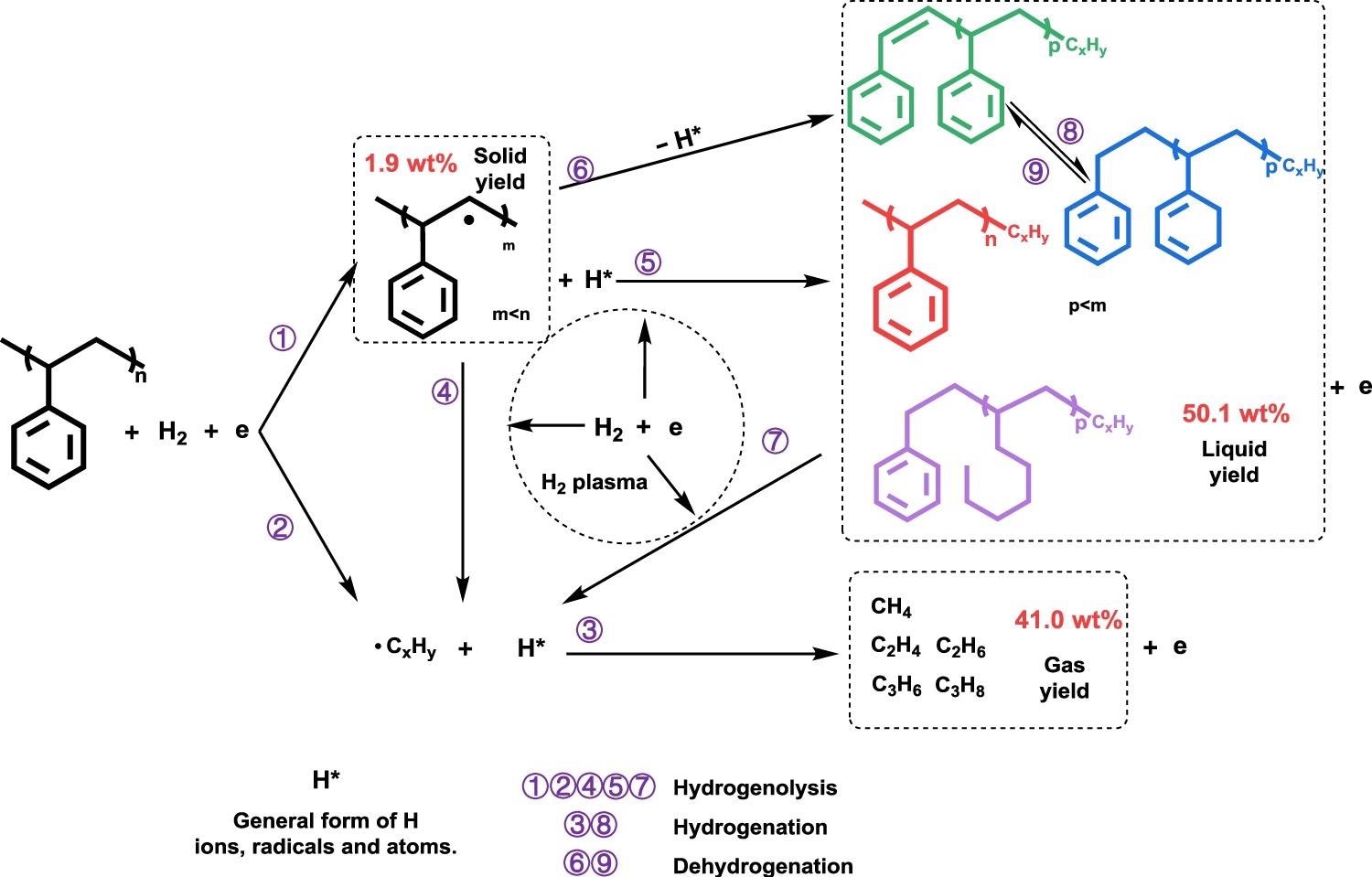
Illustrated reaction pathways for plasma-assisted PS hydrogenolysis. Product yield information shown in the figure is for PS-12 min. Image Credit: Yao, L et al., Nature Communications
Observations
The plasma-assisted hydrogenolysis reaction resulted in the generation of all three states of products viz. solid, liquid, and gas, with a mass recovery of 93%. The time-dependent product distribution graph showed a steady rise in the gas products yields up to 41.0% after 12 min of reaction time. The liquid product yield was in a range of 50-56%. Meanwhile, only 5% of yield products were in solid-state after 12 min of reaction time.
Furthermore, the majority of gas products were light-weight hydrocarbons (i.e., C1-C3). At the beginning of the reaction, methane (CH4) had the highest selectivity of 53.2 wt%, which changed to ethylene with more than 70% selectivity by the end of the time of 12 min. This was equivalent to a total ethylene yield of 38 wt%.
The nuclear magnetic resonance (NMR) analysis showed that the liquid products extracted from a liquid-solid mixture using CHCl3 extractant were primarily composed of styrene-based monomers and oligomers. The PS samples consisted of two types of molecular weights (Mw) i.e. 119,000 Da and 1700 Da. The Mw of the high-density PS decreased by a factor of 10 after the 12 min of hydrogenolysis reaction.
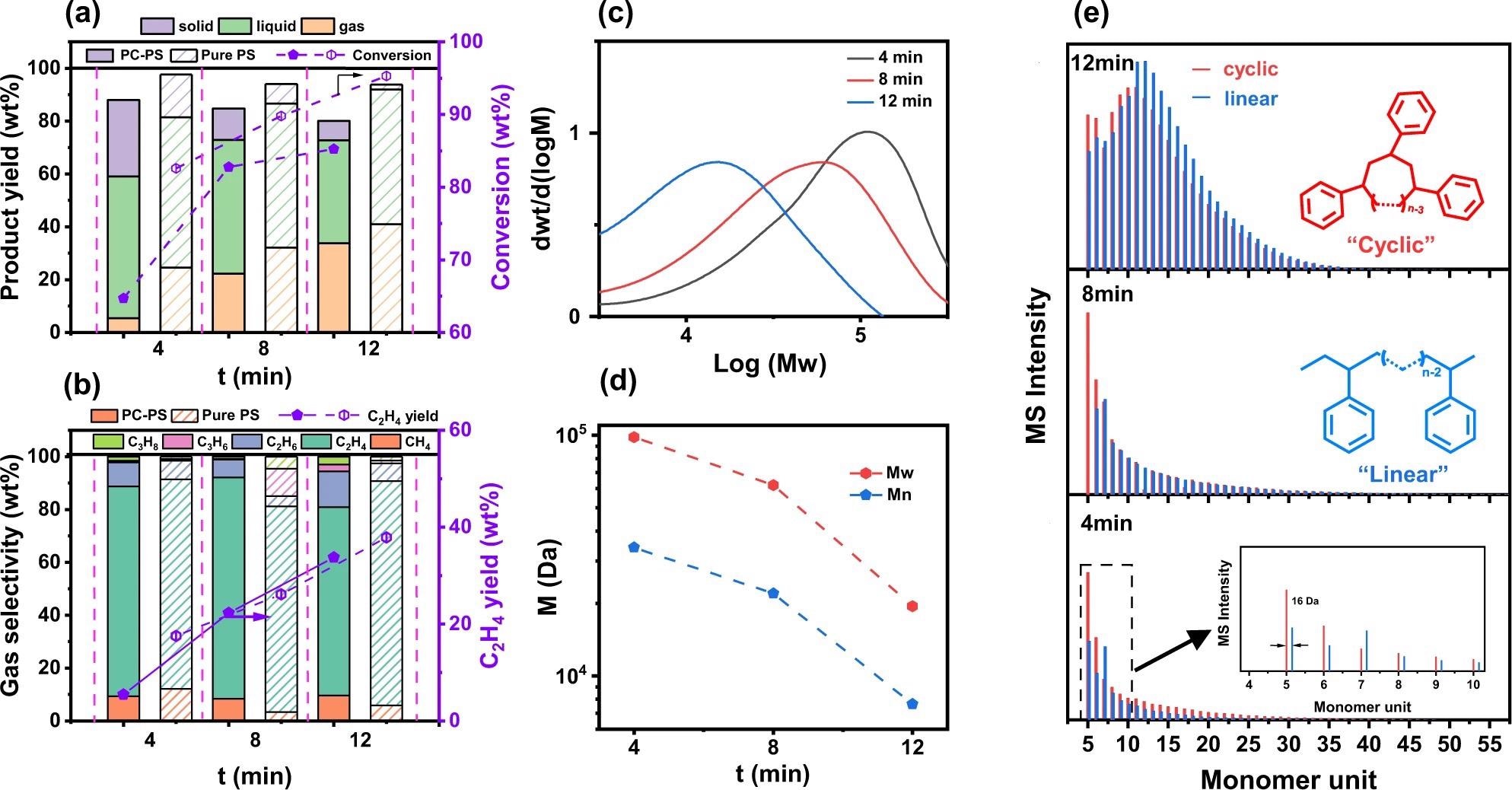
Plasma-assisted post-consumer PS (PCPS) hydrogenolysis properties as a function of reaction time. a Product yield and conversion properties. b Gas products selectivity and ethylene yield properties. c, d GPC spectra and molecular weight evolution as a function of reaction time. e MALDI-MS spectra of liquid components obtained from PCPS-4min, PCPS-8min and PCPS-12 min, respectively. Cyclic and linear polystyrenes were represented in red and blue, respectively. Image Credit: Yao, L et al., Nature Communications
Conclusions
To conclude, the researchers of this study analyzed an H2 plasma-assisted hydrogenolysis reaction at ambient temperature and atmospheric pressure for recycling PS PWs. The processes efficiently depolymerized 93 wt% of PS mass into low Mw hydrocarbons mostly with a carbon crosslinking of C1-C3. It showed a high selectivity of more than 70% to ethylene over any other hydrocarbons, which was equivalent to 38 wt% of total yield. Hence, this method can pave the way for rapid hydrogenolysis reaction-based recycling of other PWs as well
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.
Source:
Yao, L., King, J., Wu, D., Ma, J., Li, J., Xie, R., Chung, S., Miyoshi, T., Peng, Z., Non-thermal plasma-assisted rapid hydrogenolysis of polystyrene to high yield ethylene. Nature Communication, 13, 885 (2022). https://www.nature.com/articles/s41467-022-28563-7