Researchers in Poland have developed an innovative elastomer for use in heart assistance devices, with the results of their research demonstrating good potential for its use in medical applications. The research has been published online this week in the journal Polymers.

Study: In Vivo Biocompatibility of an Innovative Elastomer for Heart Assist Devices. Image Credit: Anastasiia Burlutskaia/Shutterstock.com
Medical Devices and Material Requirements
Medical devices used for implants and other applications require the materials used in their manufacture to meet stringent requirements in terms of performance and safety. Chief amongst these is their biocompatibility and lack of toxicity to the patient. Devices are subjected to biological evaluation and biocompatibility studies to assess their interaction with bodily tissues, cells, or body fluids.
Biocompatibility requirements for devices and their materials are governed by the ISO 10993 standard. Tests are chosen based on the type of device, its materials, and its intended use. Alongside this, tests are chosen regarding how long the device will stay in contact with the patient’s body as well as the nature of that contact. Tests may include evaluations of cytotoxicity, hemocompatibility, genotoxicity, and assessing local reactions post-implantation, as well as other requirements.
Polyurethanes
Poly(urethanes) are a class of polymers that are amongst the most attractive groups of non-degradable materials for biomedical applications. These polymers have been used in multiple medical devices, including pulsed heart assist pumps, mechanical leaf valves, and artificial hearts. They are particularly useful for devices that are in contact with blood.
The main advantages of these materials are their hemocompatibility and biostability. However, they can possess the tendency to creep, which makes their use challenging for long-term use. Minor surface degradations in these polymers can improve their adherence, but they can also provide places where bacteria can accumulate, increasing the risk of infection during implementation and long-term use.
The search for new, improved biocompatible polymers which can be used to fabricate medical devices is a key area of medical research. There is a growing body of studies that aim to find materials that meet the performance requirements of pulsed blood pumps that can function over a long period under cyclical loads.
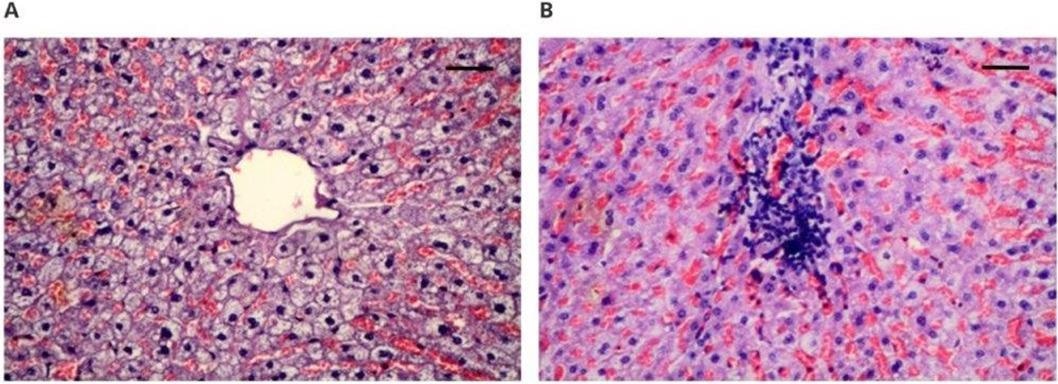
Microscopically assessed image of the heart muscle obtained from implanted animals: (A) 100× magnification (scale bar 100 µm), hematoxylin–eosin staining magnification; (B) 100× magnification (scale bar 100 µm), Masson’s staining. Image Credit: Zawidlak-Węgrzyńska, B et al., Polymers
The Research
The research published this week has demonstrated the development of PET/DLA, which is a composite biopolymer consisting of polyethylene terephthalate and a D-glucitol modified dimer fatty acid. It is intended for initial application in the Polish health system to fabricate heart support systems for both adults and children (ReligaHeart EXT and ReligaHeart PED, respectively.)
Preliminary studies of the proposed biopolymer indicated that it is resistant to biodegradation and possesses no hemolytic activity. Moreover, its atrombogenic properties are comparable to commercially available polyurethane, which is currently used in medical devices, particularly heart assistive devices.
Previous work by the authors was expanded on in this study. In their previous research, the authors assessed the stability and cytotoxicity of the biocomposite polymer in synthetic bodily fluids as well as its hemolytic and thrombogenic properties. Acceptable levels of biocompatibility were demonstrated in the previous work, with low hemolytic activity and thrombogenicity in mouse models. Moreover, there was no observable harm to human erythrocyte cells.
The authors have stated in the study that confirmation of biocompatibility for use as a long-term heart prosthesis is a necessary next step in their research. Aside from heart assistive devices, other research has demonstrated the suitability of PET/DLA as a drug delivery system, with the possibility of use for the treatment of ovarian cancers.
Implanted devices must also lack the danger of inducing inflammatory responses in the patient. This is one of the main causes of implant failure and leads to negative clinical outcomes for individuals. Inflammatory responses include cell death, osteolysis, and cell proliferation. Animal models can be employed to elucidate experimental data on the risks of inflammatory responses. The novel biocomposite was evaluated in comparison to clinical-grade, commercially available polyurethane.
The materials' chemical stability was assessed post-implantation and euthanasia using FTI, gel permeation chromatography, and scanning electron microscopy. Statistical analysis was performed.
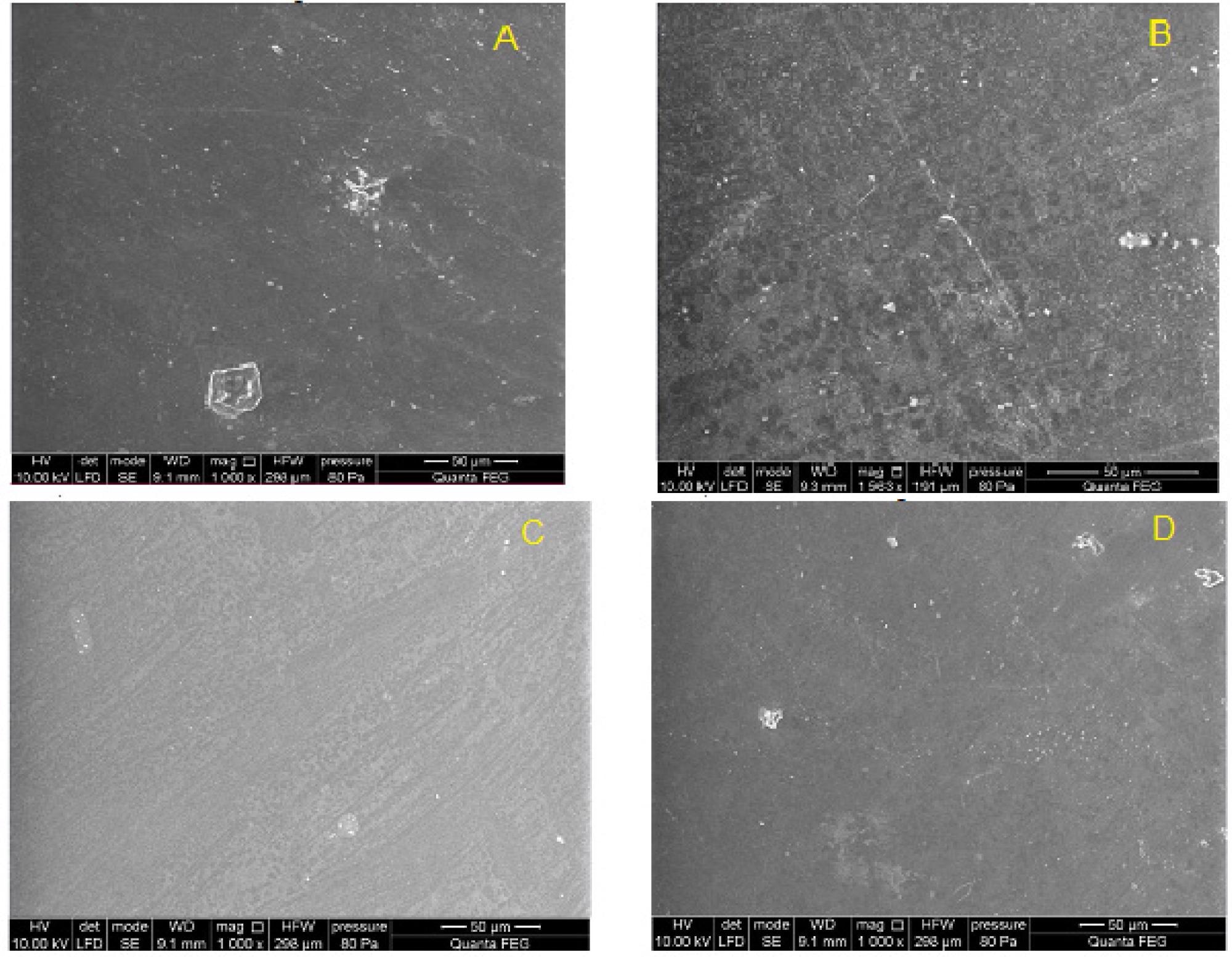
Surface of PET-DLA 70 copolymer: (A) not implanted, and (B–D) after 12-week implantation period. Image Credit: Zawidlak-Węgrzyńska, B et al., Polymers
Observation Results
Monitoring of inflammatory responses in the patient after implantation of devices using PET/DLA demonstrated normal surgical healing with no inflammatory response in the patients. Blood count and blood smear results were obtained by the authors. Additionally, they gathered information on the concentration of CRP, an inflammatory marker. The authors noted no significant differences in parameter values during their observation of the post-op inflammatory response.
No pathological effects at the implantation site or organs were observed. CRP levels, peripheral blood counts, and observations of control animals were observed to be normal. Histopathological examination of tissues and organs (kidneys, heart, spleen, liver, lungs) demonstrated acceptable biocompatibility of PET/DLA. Observations of a lack siderophages and interstitial fibrosis in the lungs of euthanized animals demonstrated that the changes resulted from euthanasia and not the implant.
Chemical stability was assessed to be acceptable for the biocomposite polymer material in the presence of biological structures. The results demonstrate a highly biocompatible material for medical implants with good chemical stability. However, the authors have noted that the material requires final applicability verification, which should be carried out using prototype heart-assistive devices in large animals.
Further Reading
Zawidlak-Węgrzyńska, B et al. (2022) In Vivo Biocompatibility of an Innovative Elastomer for Heart Assist Devices [online] Polymers 14(5) 1002 | mdpi.com. Available at: https://www.mdpi.com/2073-4360/14/5/1002
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.