 By Surbhi JainReviewed by Susha Cheriyedath, M.Sc.Mar 9 2022
By Surbhi JainReviewed by Susha Cheriyedath, M.Sc.Mar 9 2022In an article recently published in the open-access journal Materials, researchers discussed the in vitro toxicity of bone grafting materials to human mineralizing cells.

Study: In Vitro Toxicity of Bone Graft Materials to Human Mineralizing Cells. Image Credit: Tyler Olson/Shutterstock.com
Background
Bone grafting procedures are commonly used in surgical dentistry to replace lost teeth with dental implants. A wide range of bone grafting materials and processes have been developed and tested. In comparison to autogenous bone grafts, which have a high resorption rate, xenografts have been used in clinical practice, displaying a good survival rate of dental implants.
The impact of compressive stresses on calvaria defects and extraction sites after grafting has been studied in recent investigations. However, in order to enhance the clinical outcomes of this type of grafting, it is necessary to evaluate the osteogenic capacity of bone grafting materials as well as their potential toxicity in vitro. Bone chips are frequently utilized as fillers to reinforce dental implants and aid bone integration. Tungsten is a frequent contaminant associated with bone, and new in vivo studies have shown toxicity in mice.
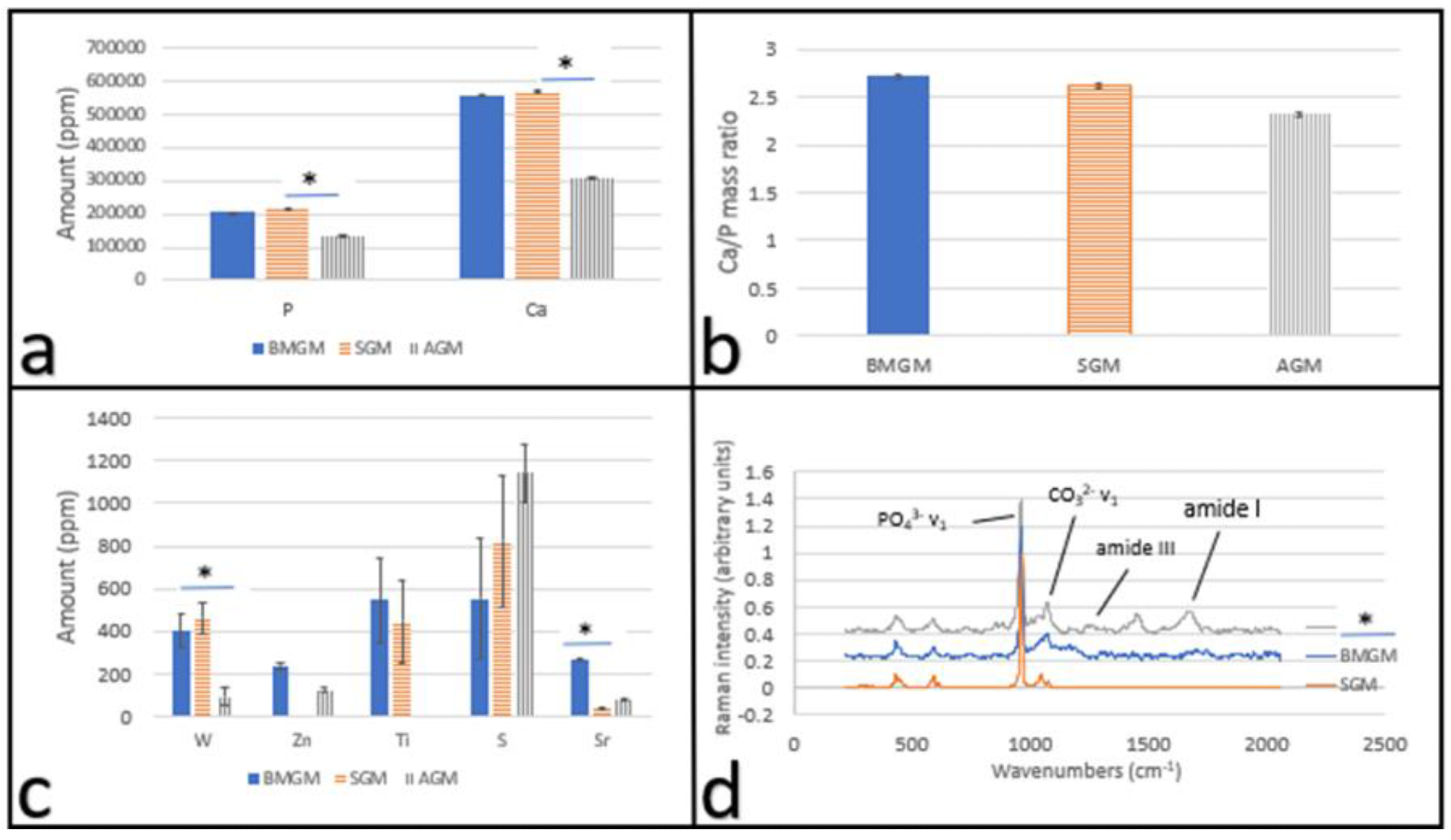
Characterization of bone graft materials: X-ray fluorescence of the bone chip samples (a) Hydroxyapatite: Ca and P amounts and (b) Ca to P ratio; (c) other trace metal impurities; * indicates p << 0.01 and (d) Raman spectra of the bone chip samples showing the amine and hydroxy apatite peaks. Image Credit: Yang, F et al., Materials
About the Study
In the present study, in vitro cytotoxicity testing was performed on dental pulp stem cells (DPSCs) and osteosarcoma cells using bone graft materials from synthetic, bovine, and human sources (Saos-2). The amount of collagen present in the bone-derived materials and the corresponding mineral to protein ratio were evaluated. Tungsten values in the bovine, synthetic, and human bone chips were determined. The doubling times of these chips applied to DPSCs on tissue culture plastic were also estimated.
The effect of using bovine or allogeneic graft materials on the cell counts was also illustrated. Tungsten was linked to cytotoxicity since only the concentration in human bone chips was below 184 ppm. The tungsten concentration in commercially available bone chips was studied, as well as the short-term effects on the stem cells of the human dental pulp and osteosarcoma bone cells cultivated on tissue culture plastic and collagen, which was a major component of bone's natural extracellular matrix.
The amount of Ca and P was determined in the proposed materials, as well as the Ca/P ratio was determined using XRF analysis. A statistically significant number of additional contaminants in the samples were discovered using X-ray fluorescence (XRF).
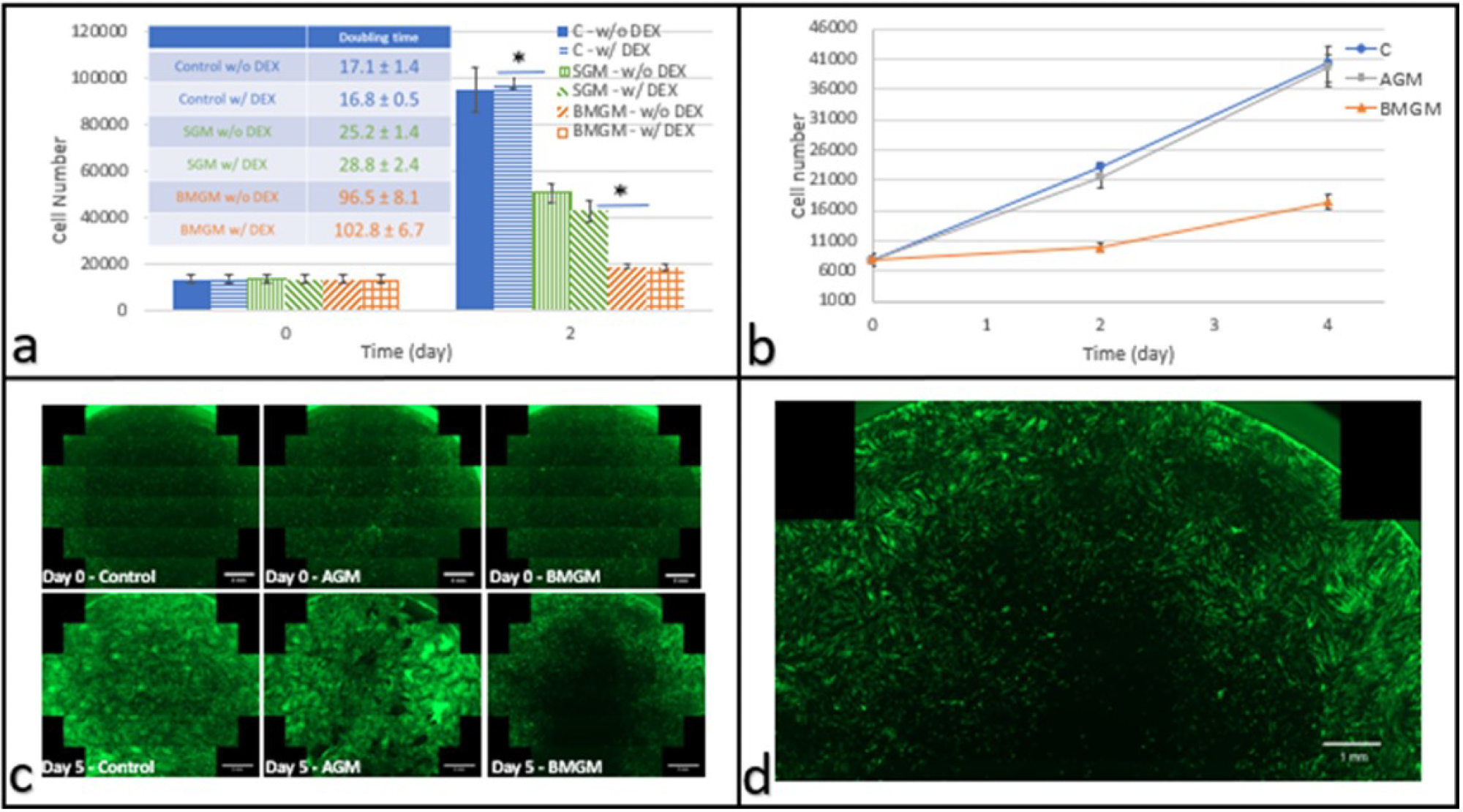
DPSCs cultured with bone graft materials on tissue culture plastic: (a) Cell numbers on days 0 and 2 after plating with and without DEX (n = 3) Inset: Corresponding doubling times (hours), with and without DEX. (b) Cell counts as a function of time in culture (w/o DEX) where * indicates p << 0.01 (c) fluorescence images of the entire well of eGFP DPSC (scale bar: 2 mm) at days 0 and 5 after addition of AGM or BMGM; Control wells received no additions. (d) Magnified fluorescence image of the well containing cells cultured with BMGM for 5 days (scale bar: 1 mm) showing a zone of exclusion around the region where the bone grafts were placed. Image Credit: Yang, F et al., Materials
Observations
Only human bone-derived materials showed significant amounts of collagen in Raman spectroscopy, with a mineral to protein ratio of 3.55 ± 0.45. In synthetic, bovine, and human bone chips, XRF indicated tungsten (W) values of 463 ± 73, 400 ± 77, and 92 ± 42 ppm, respectively.
After two days, the doubling times of these chips applied to DPSCs on tissue culture plastic were the same as the controls. It was 96.5 ± 8.1 h for those cultivated with synthetic chips and 25.2 ± 1.4 h for those cultured with bovine chips, respectively. Saos-2 had a higher sensitivity. Cell counts decreased within the first two days after using allogeneic or bovine graft materials. Allogeneic and bovine bone chips had no effect on DPSC doubling times when cultured on collagen.
None of these contaminants were found to be hazardous to human cells at ppm levels, with the exception of tungsten, which was demonstrated to be toxic at concentrations exceeding 184 ppm. Both the bovine (BMGM) and synthetic (SGM) bone chips exceeded this quantity, with 400 ± 77 ppm and 463 ± 73 ppm, respectively; however, the human bone chips had a tungsten concentration of 92 42 ppm.
In the analysis of a variety of bone chips, both synthetic and animal samples contained tungsten at amounts greater than 400 ppm. In human bone chips, much lower quantities (92 ppm) were discovered. Furthermore, Raman spectra revealed that only the human bone chips had a clearly detectable amount of matrix proteins, with a mineral to matrix ratio of 3.55 ± 0.45, which was similar to that of bone, at 3.81 ± 0.42.
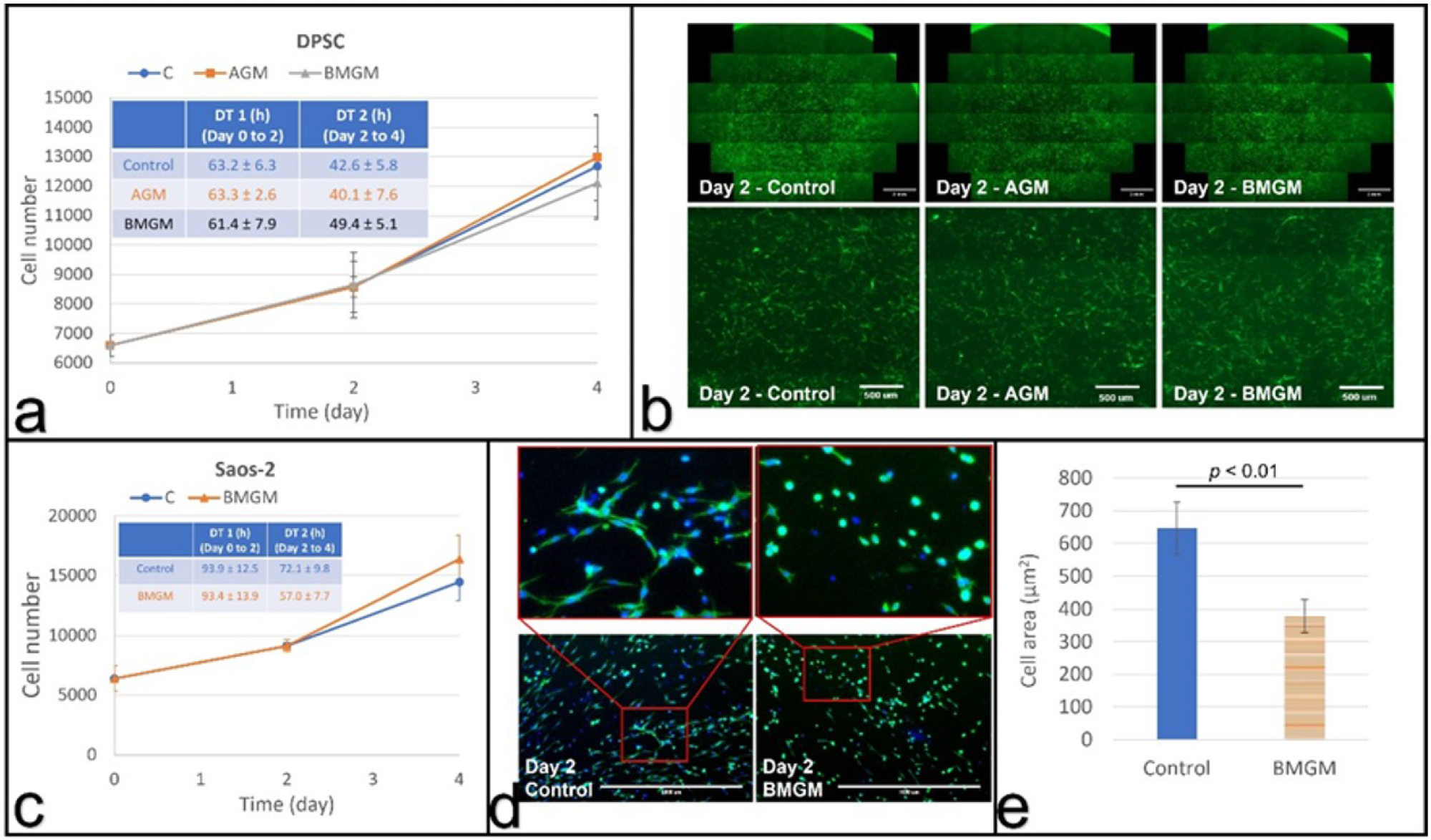
Cells cultured with bone graft materials on collagen substrates: (a) growth curves and calculated (inset) doubling times of DPSCs; (b) fluorescence images of DPSCs with the bottom row (scale bar: 500 µm) being magnified view of the area in the square shown in top row (scale bar: 2 mm); (c) growth curves and doubling times of Saos-2 cells; (d) fluorescence images of Saos-2 cells with the top row (scale bar: 1000 µm) being magnified view of the bottom row; (e) Comparison of the average cell area of Saos-2 cells at day 2. Image Credit: Yang, F et al., Materials
Conclusions
In conclusion, this study demonstrated that bone graft materials can be cytotoxic, using Saos-2 and DPSC cells as an in vitro testbed. The degree of toxicity was substantially more for the Saos-2 cells. All cells cultured on TCP showed significant toxicity on BMGM and SGM. On DPSC, allograft graft material (AGM) showed no toxicity, whereas Saos-2 started showing toxicity by day two which was followed by a partial recovery by day four.
The fluorescence images revealed zones of exclusion around the graft materials, which had lower or no cell numbers. The toxicity of both types of cells is reduced when they are plated on collagen substrates. It was also observed that the bone chips are closely packed in vivo, and significantly larger concentrations may be present, highlighting the need for caution when using these materials in practice. Overall, the authors believe that these findings demonstrate the importance of the exploration of dental materials.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.
Source:
Yang, F., Li, K., Fu, S., et al. In Vitro Toxicity of Bone Graft Materials to Human Mineralizing Cells. Materials 15(5), 1955 (2022). https://www.mdpi.com/1996-1944/15/5/1955