 By Surbhi JainReviewed by Susha Cheriyedath, M.Sc.Mar 10 2022
By Surbhi JainReviewed by Susha Cheriyedath, M.Sc.Mar 10 2022In an article recently published in the open-access journal Coatings, researchers discussed the effect of graphene oxide (GO) additions on a rust converter primer's corrosion resistance.

Study: Influence of Graphene Oxide Additions on the Corrosion Resistance of a Rust Converter Primer. Image Credit: olympuscat/Shutterstock.com
Background
One of the most prevalent ways for preventing corrosion is to employ paints to protect steel structures. The success of this method is highly reliant on the metal's surface preparation. The ideal state is for all rust to be removed; however, this is not always possible due to a variety of factors such as inaccessibility to the elements, the need to repair massive structures where typical rust cleaning methods are too expensive, or environmental limits.
In these cases, stabilizing the oxide layer prior to painting with rust converters, which are chemical formulations capable of transforming iron oxides into a more compact and adhering coating, is an appealing alternative.
The most frequent formulations include tannic or phosphoric acids as active ingredients, with gallic acid being added more recently. Even though they are environmentally friendly alternatives to pollutant pre-treatments, their efficacy is debatable. Incorporating corrosion inhibitors into commercial rust converter (RC) could be a viable solution, following the same path as traditional paint compositions.
Functionalization of GO is a popular strategy for improving GO's compatibility with the polymeric matrix and avoiding difficulties like dispersion and agglomeration. However, the experimental technique is usually complicated, limiting its application on a large scale.
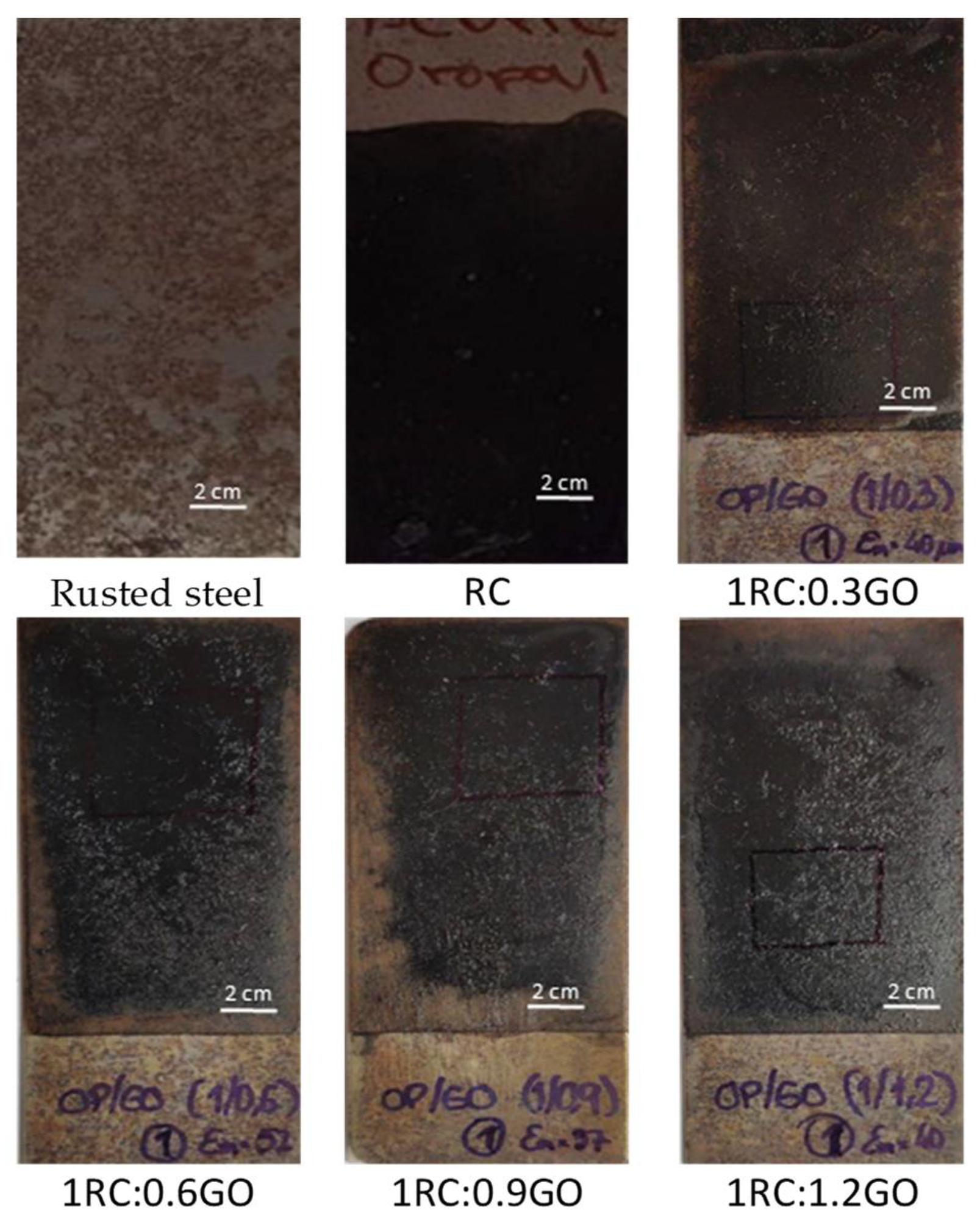
Pictures of rusted steel samples that were coated using the different RC:GO ratios, with 80 mm × 30 mm × 1 mm dimensions. Image Credit: Diaz, B et al., Coatings
About the Study
In the present study, the author investigated the performance of a commercial RC doped with five distinct RC:GO ratios, namely 1:0, 1:0.3, 1:0.6, 1:0.9, and 1:1.2 (% v/v). The effect of RC and RC + GO additions in iron oxides was shown using the X-ray diffraction (XRD) technique.
To determine the surface charge of the particles of GO, zeta-potential measurements are performed. The corrosion resistance of rusted samples covered with five different rust converter formulations was also investigated. To determine the best formulation range, different quantities of GO were added to a commercial RC.
The experimental results were explained using the electrochemical impedance spectroscopy (EIS) technique and an electrical equivalent circuit. The GO was added as an aqueous dispersion over commercial water-based RC, making the mixing process easier and the mixture dispersion good. The primary focus of this work was to optimize the GO concentration that provided the optimum anti-corrosive capabilities of the commercial rust converter.
Zeta potential measurements were used to assess the surface charge of graphene oxide particles in interaction with gallic acid. Using the Smoluchowski model, the zeta potential was derived using electrophoretic mobility, with the thickness of the electrical double layer assumed to be minimal in relation to particle size. The rust converter's interaction with iron oxides was studied using the XRD technique. EIS technique was used to evaluate the protective properties of the RC/GO formulations applied to corroded steels.
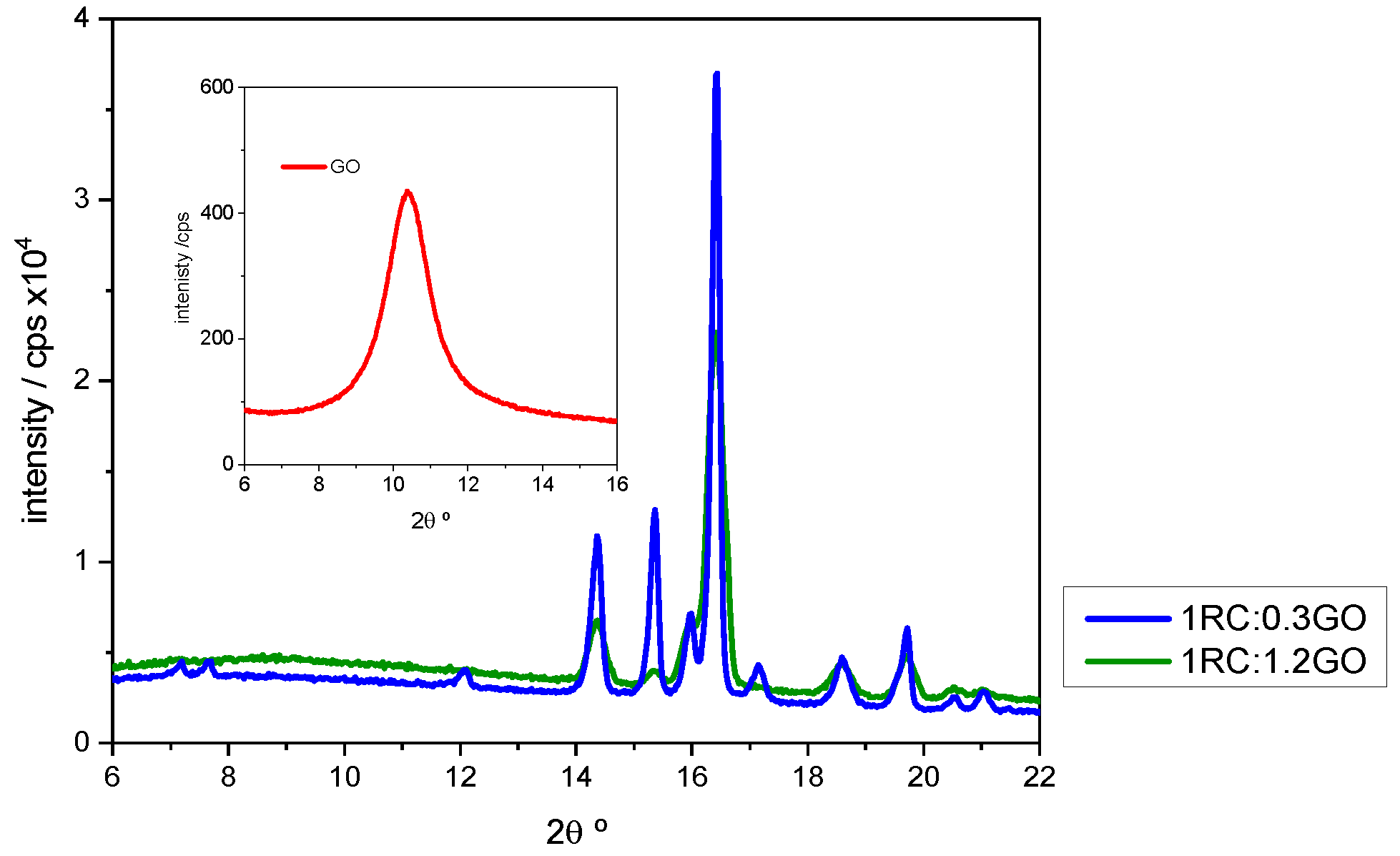
Detail of the X-ray patterns corresponding to 1RC:0.3GO and 1RC:1.2GO ratios. The X-ray pattern of GO is given in the insert. Image Credit: Diaz, B et al., Coatings
Observations
The ideal RC:GO ratio was found to be between 1:0.3 and 1:0.6. It was observed that the higher the RC:0.3GO ratio, the greater the corrosion resistance. The zeta-potential results supported the stability of the colloidal suspensions for all RC:GO ratios and the interaction between gallic acid and GO resulted in a reduction in the surface charge of the particles of GO.
This interaction was also confirmed by XRD, which showed that when the RC:GO ratio increased, the strength of the peaks associated with gallic acid reduced significantly. The XRD technique also demonstrated the primary crystalline oxide/hydroxide complexes found in corroded steel. Lepidocrocite -FeO(OH) and magnetite Fe3O4 were the two detected minerals.
EIS illustrated a comparative evaluation of the protective properties of all formulations applied to corroded steel. The impedance dropped with immersion duration in general, except for the 1RC:0.3GO ratio, where the impedance increased with time. The 1RC:1.2GO and 1RC:0.9GO ratios yielded lower impedance levels. The dielectric characteristics of the conversion layer were linked to the high-frequency time constant, the corrosion process to the middle-frequency time constant, and the oxygen transport through the layer pores to the low-frequency time constant. The 1RC:0.3GO ratio performed better as compared to the 1RC:0.6GO formulation.
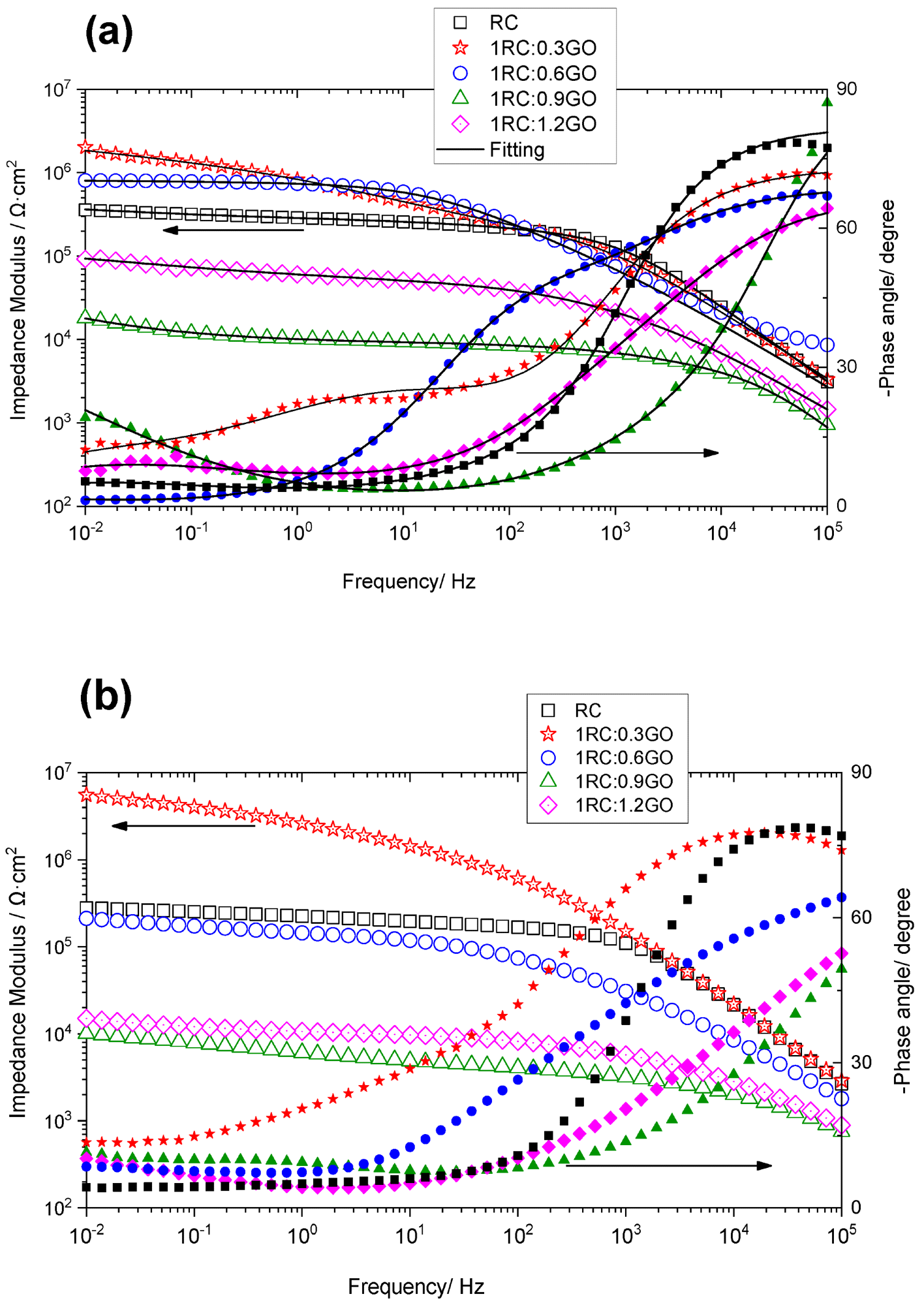
Impedance modulus (open symbols) and phase angle (filled symbols) of the rusted steel coated with the RC/GO formulations after (a) 4 days and (b) 21 days of immersion in 0.06 M NaCl solution. Image Credit: Diaz, B et al., Coatings
Conclusions
In conclusion, this study demonstrated the utility of GO nanoparticles in polymers to improve corrosion resistance. The advantage afforded by modest levels of GO was explained by considering its 2D shape, which boosted the barrier characteristics.
The authors emphasized that gallic acid has the potential to partially lower the hydrophobic GO (rGO). They also observed that agglomeration difficulties can occur if the GO concentration is too high, resulting in a more porous layer. They believe that higher levels of rGO can create a large cathodic surface, which can speed up the corrosion process.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.
Source:
Diaz, B., Novoa, X. R., Perez, C., et al. Influence of Graphene Oxide Additions on the Corrosion Resistance of a Rust Converter Primer. Coatings 12(3), 345 (2022). https://www.mdpi.com/2079-6412/12/3/345