Researchers from Italy and Spain have developed photocurable hydrogels derived from chitosan and gelatin to treat water and remove contaminants. Their work has been published online in the journal Polymers this week.

Study: UV-Cured Chitosan and Gelatin Hydrogels for the Removal of As(V) and Pb(II) from Water. Image Credit: People Image Studio/Shutterstock.com
Removing Contaminants from Water Supplies
Access to safe and sanitary drinking water is essential for human society. The rapid growth in urbanization and industrialization has severely affected water quality, leading to contamination on a global scale.
There are numerous organic and inorganic contaminants that are caused by domestic and industrial activities, including pathogens and industrial chemicals. Amongst the various contaminants that enter global water supplies, heavy metals such as lead are of particular concern due to the environmental and health damage they cause.
Heavy metals such as copper, lead, zinc, arsenic, and nickel can come from both natural (for example, volcanic emissions) and anthropogenic (for example, pharmaceutical release, mining, the electronics industry, and agriculture) sources. These materials are particularly problematic contaminants due to their poor biodegradability, bioaccumulative effects, and high toxicity. Once they are released into the environment, they can enter the food chain, proliferating in fish, shellfish, and other marine food sources, leading to health problems in humans.
The critical nature of heavy metal contamination has led to regulations to limit the release of these toxic materials into the environment. Additionally, these contaminants are difficult to completely remove from water supplies, meaning that the WHO has set limits for the safe contents of the water used for drinking and sanitation.
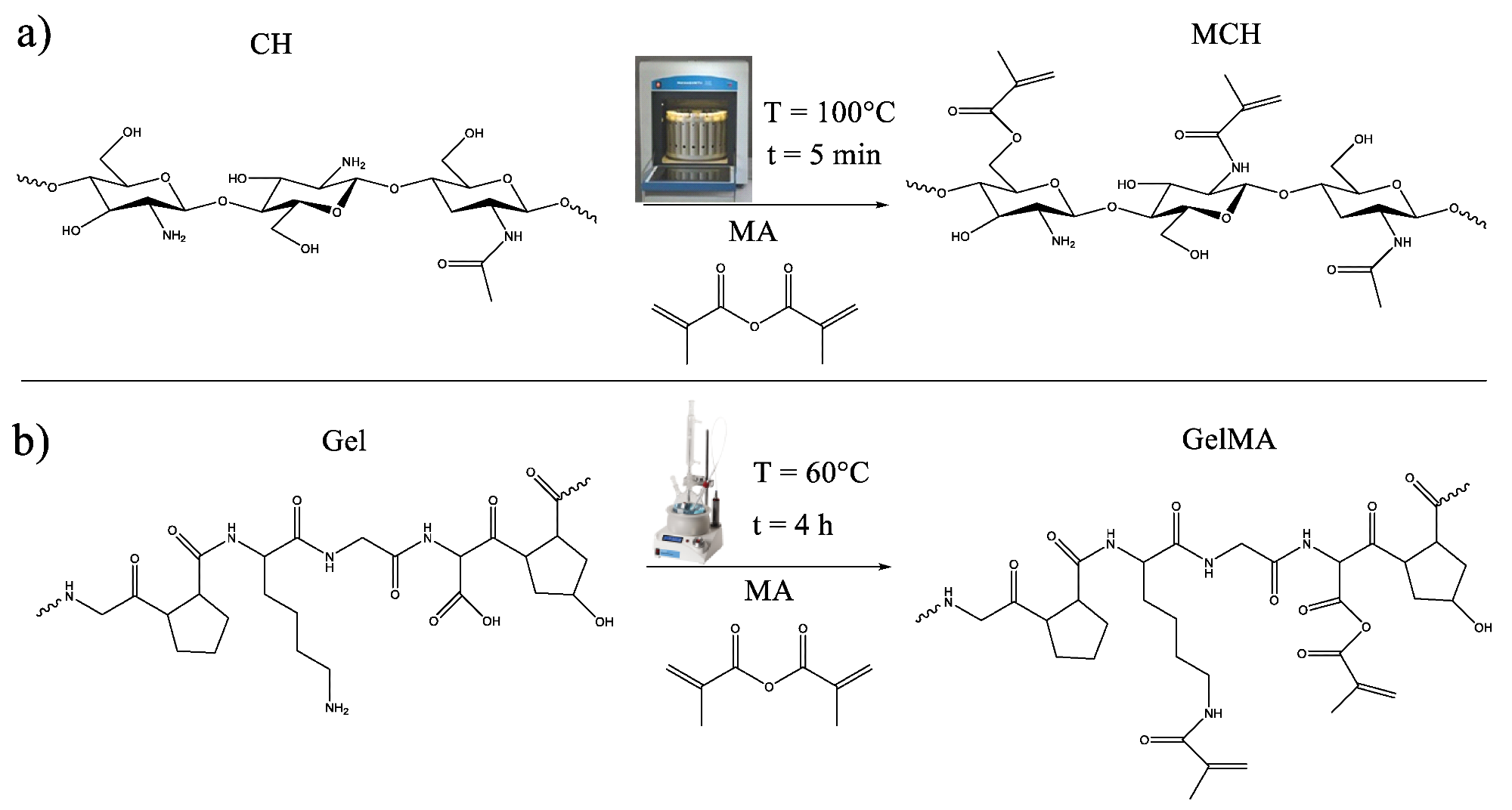
Scheme of the methacrylation reaction of (a) chitosan and (b) gelatin. Image Credit: Noè, C et al., Polymers
Heavy Metal Contamination Remediation Strategies
Removing heavy metal contamination from wastewater is vital to ensure safe and sanitary drinking water and protect the environment. Strategies widely proposed and developed for the removal of these contaminants over the past few decades include membrane filtration, ion exchange, photocatalysis, and adsorption.
Amongst these strategies, adsorption has been proposed as a viable solution for industrial-scale water treatment, due to its superior efficiency for removing heavy metals from wastewater. Sorbents that have been explored for this process include carbon nanotubes, magnetic particles, silica-based materials, and activated carbon. However, these sorbents are expensive, which limits their large-scale viability.
Hydrogels overcome the issues with these sorbents and have been widely researched in recent years. Aside from being cheaper, hydrogels possess many attractive properties including high porosity, high swelling, and good water affinity. Hydrogels derived from carbohydrates and other natural materials have the added advantage of being biodegradable, non-toxic, and renewable.
Designing polymeric networks which efficiently remove different heavy metal contaminants under different conditions is essential. However, natural hydrogels have drawbacks in terms of poor mechanical performance, stability, and low adsorption capabilities. Different strategies to overcome these limitations have been proposed including chemical modification and hybrid hydrogels.
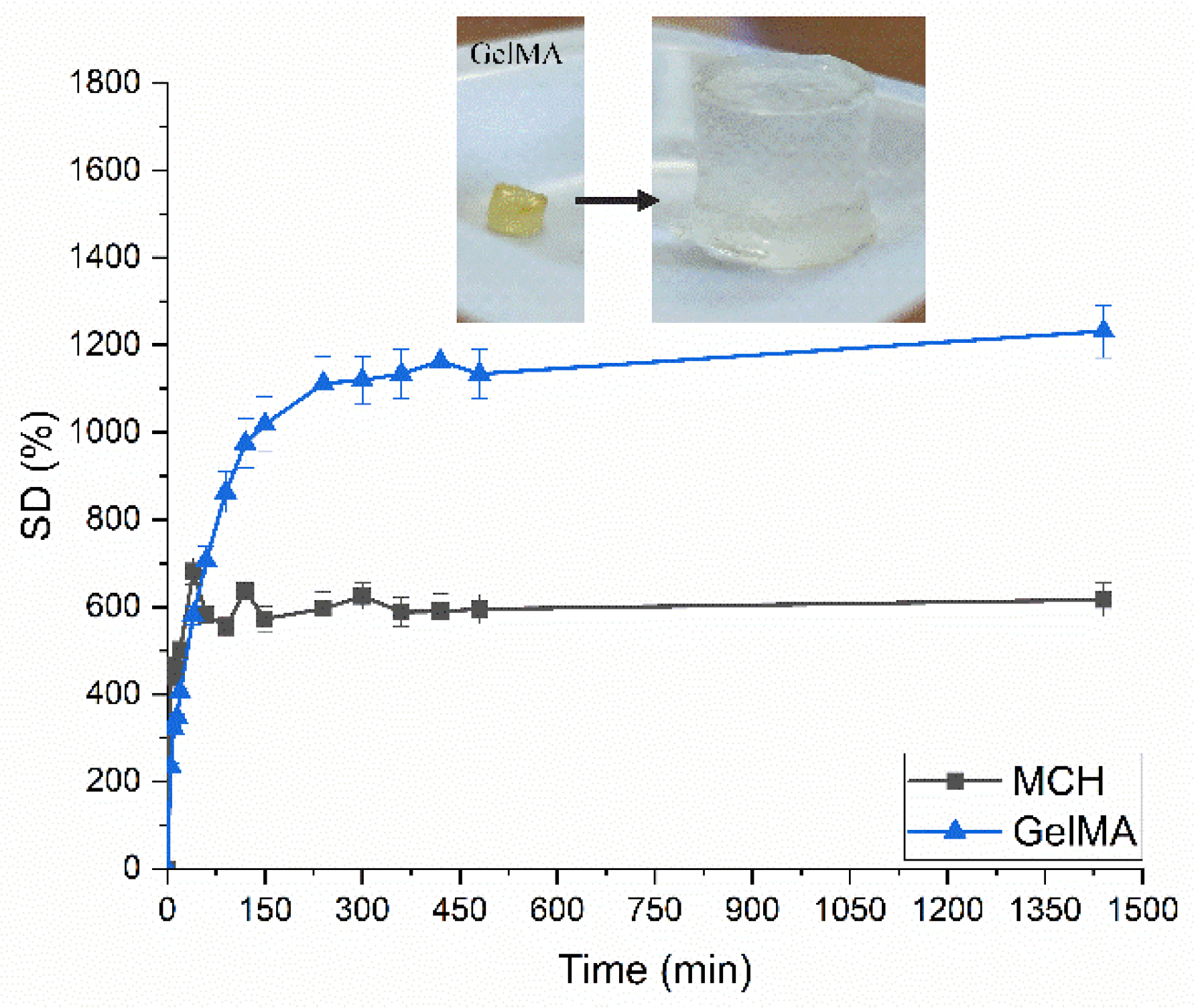
Swelling curves for UV-Cured MCH and GelMA hydrogels. In the insert is reported the swelling of GelMA UV-Cured hydrogels as an example.
The Study
A study published in the journal Polymers has investigated photo-reactive crosslinked hydrogels derived from gelatin and chitosan for the removal of heavy metal ions from wastewater sources.
Chitosan has been widely researched due to its numerous and easily modifiable reactive groups. Gelatin is a natural polymer that is biocompatible, transparent, and also contains numerous functional groups. Several crosslinkers have been explored to improve the properties of hydrogels, but these are generally toxic and must be removed before they can be used for practical applications.
The natural-based hydrogel was synthesized using methacrylation. Using this modification process, the researchers developed polymers that can be easily photopolymerized in water. Photopolymerization is an attractive process for synthesizing crosslinked hydrogels due to its fast-curing times, environmental friendliness, and room temperature processing.
The authors used FTIR spectroscopy and 1H-NMR to investigate how precursors were modified. Photo-rheology was used to evaluate the material’s curing process. The authors examined the material’s swelling capacity and investigated its correlation with the material’s adsorption efficiency. Analyzing the adsorption process’s kinetics and equilibrium and how pH influences the material’s performance provided pertinent information on the hydrogel’s adsorption for lead and arsenic, two of the most critical heavy metal contaminants.
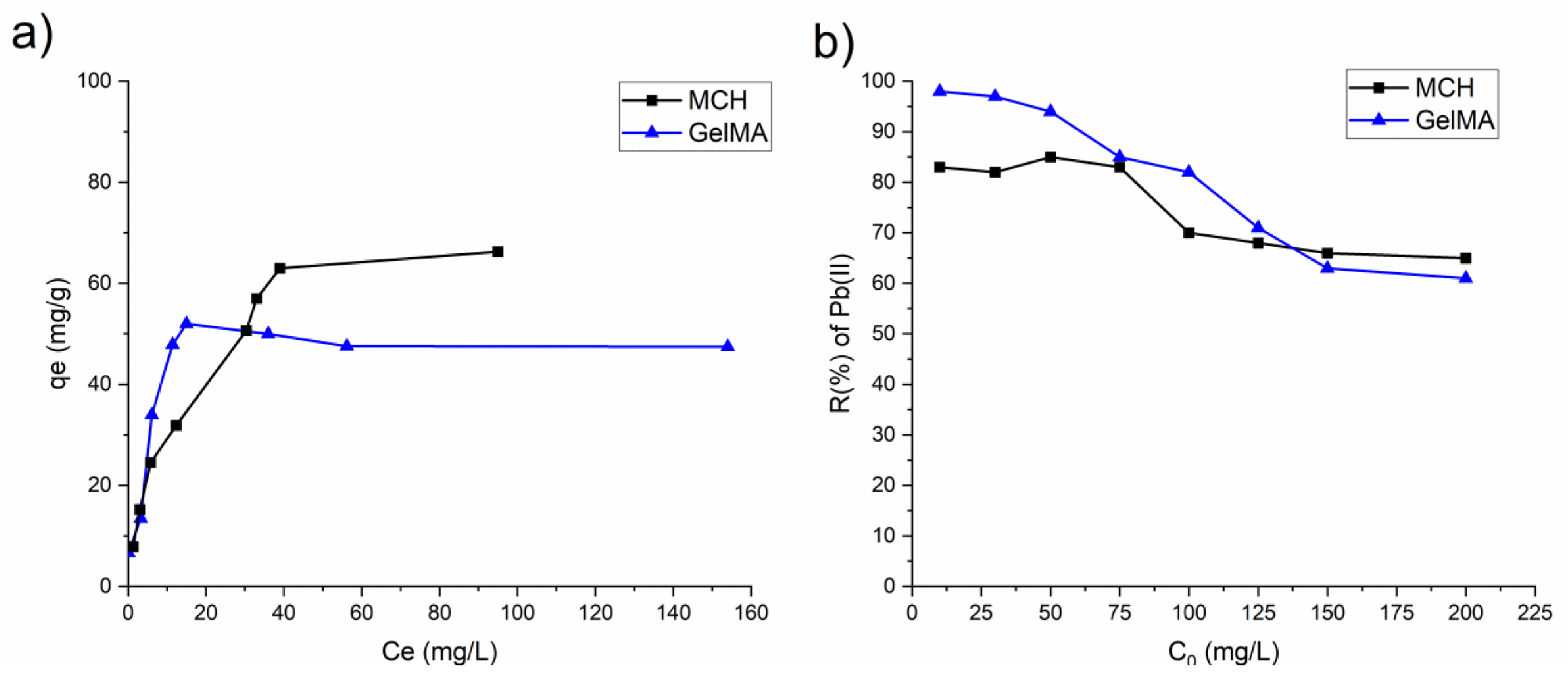
(a) Isotherm plots of MCH and GelMA hydrogels obtained from the experimental data; (b) removal percentage R(%) of Pb(II) by MCH and GelMA at different initial concentrations. Image Credit: Noè, C et al., Polymers
The authors observed that there was a noticeable influence on removal efficiency by adsorption parameters such as pH, contact time, and initial ionic concentration. Furthermore, they observed that the hydrogels possessed an extremely high swelling capacity. Photocuring times of several minutes were observed by the authors. Removal efficiencies for both arsenic and lead were extremely high.
Based on the results of their analyses and observations, the authors have stated that modified gelatin and chitosan-based photocurable hydrogels are highly efficient and can be considered attractive candidates for the removal of arsenic and lead from contaminated water.
Further Reading
Noè, C et al. (2022) UV-Cured Chitosan and Gelatin Hydrogels for the Removal of As(V) and Pb(II) from Water [online] Polymers 14(6) 1268 | mdpi.com. Available at: https://www.mdpi.com/2073-4360/14/6/1268
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.