A team of researchers from Spain, France, and Australia has highlighted the development and advantages of double-layer polymer electrodes in lithium-ion batteries. Their research has been published in the journal ACS Energy Letters.

Study: Toward High-Voltage Solid-State Li-Metal Batteries with Double-Layer Polymer Electrolytes. Image Credit: Black_Kira/Shutterstock.com
Lithium-ion Batteries
Lithium-ion batteries have emerged as a key technology in the search for renewable, clean energy solutions. These devices are used to power nearly every portable electronic device currently in use, and the drive toward electrification in medium- and large-scale industrial applications has led to research into materials and chemistries which facilitate the development of lithium-ion batteries with higher energy thresholds.
Amongst the currently proposed technologies in the field of lithium-ion battery research, solid-state lithium-metal batteries, which have solid polymer electrolytes, have proven promising. These devices can provide higher energy densities than conventional batteries and have improved safety. By replacing the liquid electrolyte with a solid polymer electrolyte, more efficient cell stacking can be achieved. Furthermore, high-energy Li-metal electrodes can be incorporated, which increases mechanical and thermal stability and minimizes dendrite propagation.
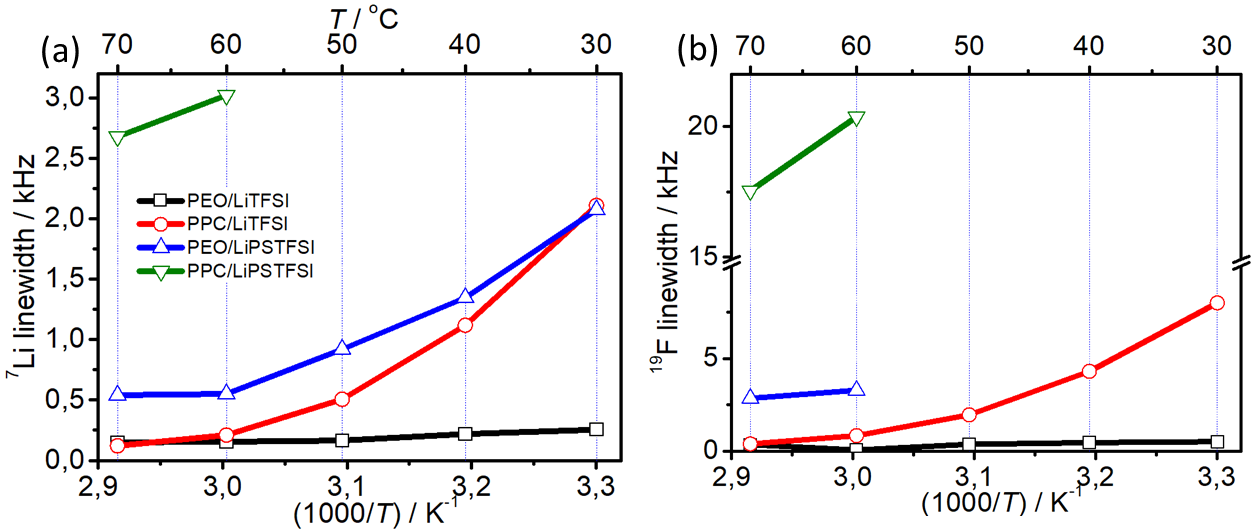
Static NMR linewidth measurements at different temperatures for (a) 7Li and (b) 19F. Image Credit: Aresse-Igor, M et al., ACS Energy Letters
Improving the Performance of Solid-state Lithium-Metal Batteries
When designing solid-state lithium-metal batteries, attention must be paid to materials selection, in particular for the solid polymer electrolytes. Materials used in different device components must meet different performance requirements. When used as electrolytes, they must be chemically compatible with lithium and provide good cathodic stability. Conversely, when used in the cathode, solid polymer electrolytes must be chemically compatible with the high-voltage active material and provide sufficient anodic stability.
So far, finding polymers that can withstand a large energy gap has proven to be challenging. Poly(ethylene oxide) has been studied extensively as a solid polymer electrolyte due to the ideal spacing of ether groups along the polymer chain and the high solvation capability of these groups. However, the ethylene oxide units can become oxidized when voltage is applied, limiting the material’s use with high-voltage cathodes.
Several approaches have been investigated to minimize the oxidation of this polymer at the battery’s positive electrode. Researchers have incorporated ceramic particles, coated active materials to improve the stability with electrolytes, investigated preventing interfacial solid-state reactions by using liquid additives, and more approaches besides. However, these approaches are insufficient as they can reduce the safety of solid-state lithium-metal batteries and deplete their high energy and power densities.
Using catholytes, which are stable at high voltages, can improve the potential of these devices. Other polymers have been proposed for this application, including poly(propylene carbonate). The poor chemical compatibility of this solid polymer electrolyte with lithium metal is a drawback to its use, but there are several other properties that make this material attractive, such as its sufficient ionic conductivity and high electrochemical stability.
Another advantage of poly(propylene carbonate) that makes it an attractive catholyte is its solubility in a wide range of organic solvents. This property allows electrolyte laminates to be prepared using slurry coating.
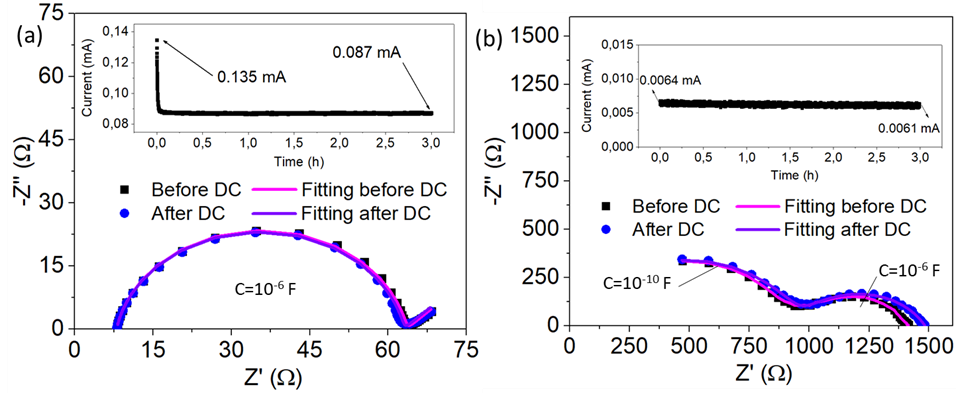
Chronoamperometry and EIS for lithium transference number estimation for (a) PEO-LiTFSI and (b) PEO-LiPSTFSI with the corresponding fitting curves. Image Credit: Aresse-Igor, M et al., ACS Energy Letters
The Study
The authors have developed a solid-state lithium-metal battery with poly(ethylene oxide) as the solid polymer electrolyte and poly(propylene carbonate) as the catholyte. The use of these polymers improves both the device’s lithium-metal stability and high-voltage capacity. The approach has been termed a double-polymer electrode. The approach is tied to careful lithium salt selection.
Using poly(propylene carbonate) as the catholyte significantly improves the cycling performance of the batteries. Additionally, smooth cycling is achieved in these devices due to higher lithium transference. The cells developed by the authors achieve over 80% capacity retention over eighty cycles. Moreover, their Coulombic efficiency is close to 100%. Overall, the double-polymer electrode approach developed by the authors creates cells with higher efficiency than currently reported devices.
The double-layer solid polymer electrolyte developed in the study was integrated into a cell with lithium-metal electrodes and LiNxMnyCO2O2 as the active material, and the use of a high-areal-capacity electrode in the device significantly improved the volumetric and gravimetric energy density.
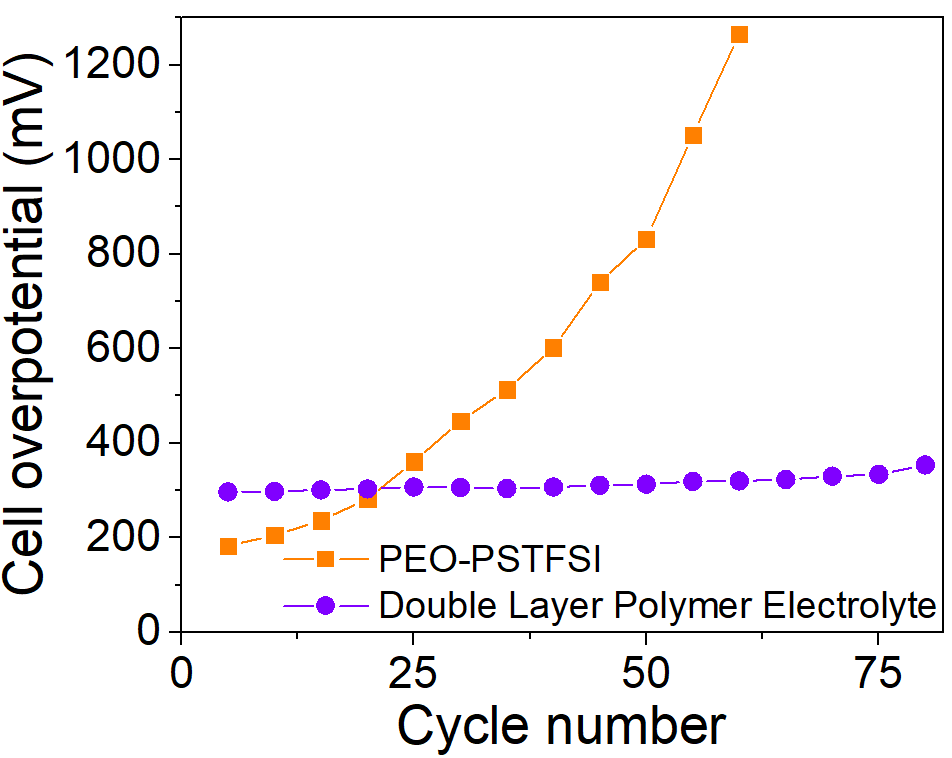
Cell overpotential evolution upon cell cycling at C/20 and 70 °C for 1 mAh·cm-2 PEO-LiPSTFSI cell and DLPE cell (estimated as the potential difference at 50% of the discharge capacity of each cycle). Image Credit: Aresse-Igor, M et al., ACS Energy Letters
The double-layer polymer electrolyte approach confers two major improvements over current technologies. Firstly, the SLIC electrolyte used in the approach ensures increased lithium-ion mobility and avoids side-reactions caused by anion accumulation at the negative electrode. Secondly, replacing poly(ethylene oxide) with poly(propylene carbonate) at the positive electrode improves stability under higher voltages, which in turn increases the cell’s stability at high potentials.
In summary, the research paper has demonstrated an innovative approach to manufacturing high-performance and stable solid-state lithium-metal batteries using a double layer of two solid polymer electrolytes. The work by the authors has promising prospects for the development of next-generation batteries for a variety of high-demand industrial applications.
Further Reading
Aresse-Igor, M et al. (2022) Toward High-Voltage Solid-State Li-Metal Batteries with Double-Layer Polymer Electrolytes [online] ACS Energy Lett. 7 pp. 1473-1480 | pubs.acs.org. Available at: https://pubs.acs.org/doi/10.1021/acsenergylett.2c00488
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.