Researchers from Harvard Medical School, the University of Heidelberg, and the Johannes Gutenberg University Mainz have investigated water-induced self-repair of pectin biopolymer films and evaluated its effects on the material’s properties. Their findings have been published in the journal Polymers.

Study: Optical and Mechanical Properties of Self-Repairing Pectin Biopolymers. Image Credit: Michelle Lee Photography/Shutterstock.com
Pectin Biopolymers
In the field of biomedical research, scientists are constantly developing new materials for a variety of applications. Amongst bio-derived materials, polysaccharide biopolymers have been the focus of research in recent years. Several natural materials have been evaluated, including cellulose, chitin, alginate, and pectin.
Pectin has shown particular promise for biomedical applications. This polysaccharide is extremely abundant and possesses unique functional and chemical characteristics. Pectin is involved in intercellular adhesion and provides plant cell walls with mechanical resistance. Recently, research has indicated that pectin also aids binding in visceral organs, potentially playing a role in healing and tissue repair.
Research into its role in binding mesothelial surfaces in organisms has highlighted pectin’s potential as a bioadhesive. Pectin adhesion is still poorly understood but appears to require a wetting phase. This wetting phase, through the interaction of a liquid interface, allows the surfaces to adhere to each other and enhances the interaction between them.
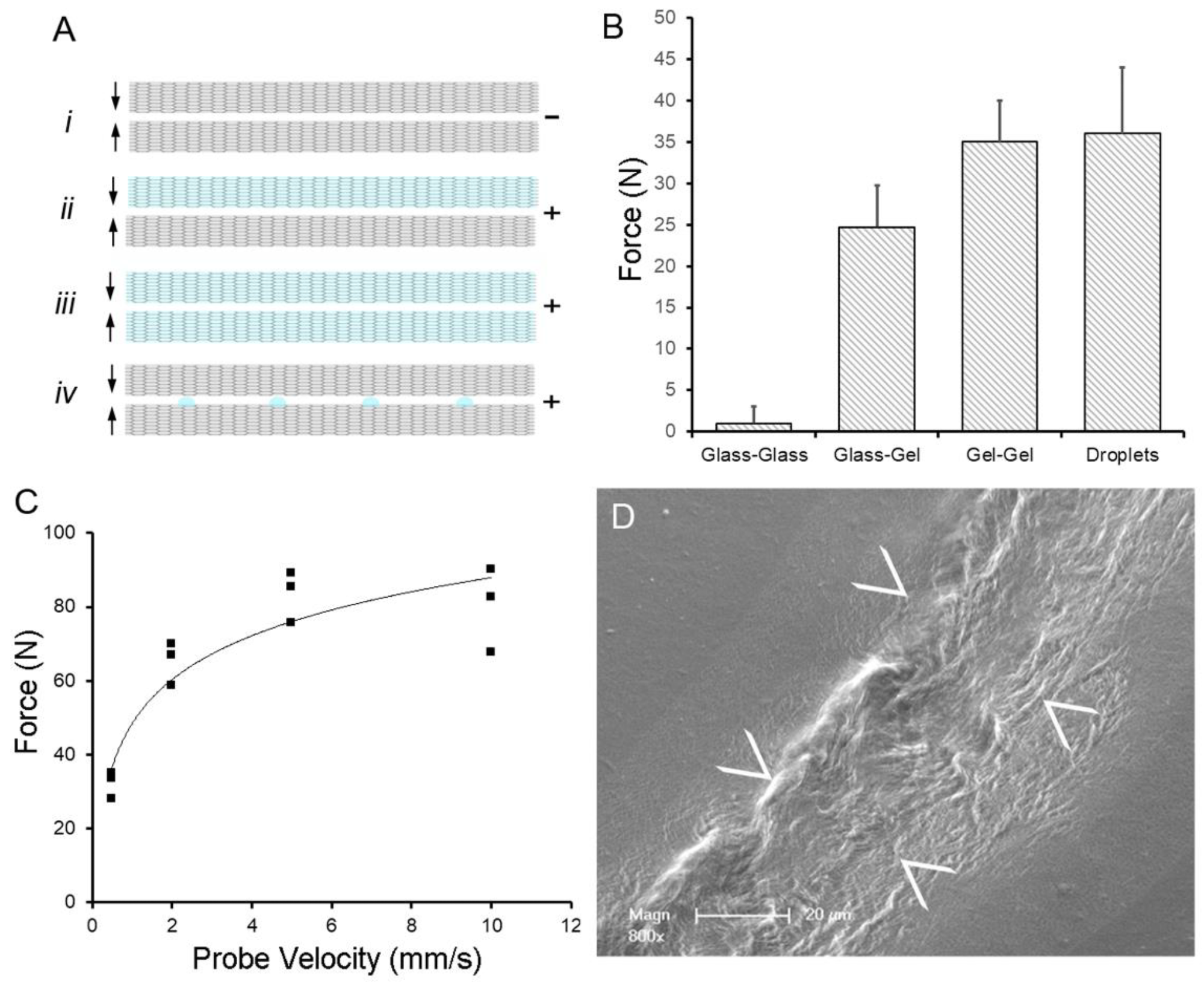
Water-induced adhesion of pectin films cured in parallel. (A) Schematic of pectin films in glass phase (gray) and gel phase (blue). The (–) indictes no adhesion, whereas (+) indicates an adhesive interaction between films. (B) Using a previously defined adhesion assay, pectin films were gently compressed (5N) for a 60 s development time followed by withdrawal of the films at 0.5 mm/s. Two glass phase films (Ai) demonstrated no adhesion. Hydration of one (Aii) or both (Aiii) of the films to 15% water content resulted in significant adhesion. An equal volume of water placed on the glass phase films as droplets (Aiv) resulted in comparable adhesion to two gel films. (C) In the droplet assay, adhesion was largely independent of probe speed (that is, the speed at which the films were compressed). (D) Scanning electron microscopy (SEM) of two films following droplet compression shows evidence of polymer chain entanglement at the film interface (arrows at film interface). Image Credit: Pierce, A.F et al., Polymers
In mucoadhesion, water is moved from the mucus layer to the pectin, which enhances inter-surface interactions. This enhances the adhesivity of pectin. Current research has indicated that entanglement of the pectin polymer’s branched chains is intimately involved in the adhesive phase. Studies have highlighted that this entanglement may have important clinical relevance.
The unique physiochemical properties of pectin have also been linked to regenerative processes in plants. This polysaccharide contributes to several important processes, such as compartmentalizing decay during tylosis. During early plant grafting, deposited pectin also appears to support cohesion and adhesion between adjacent cells. Essentially, pectin is a biological “cement” that is involved in several important functions in organisms. The polymer’s intrinsic properties may also be important for cell-free self-repair processes.
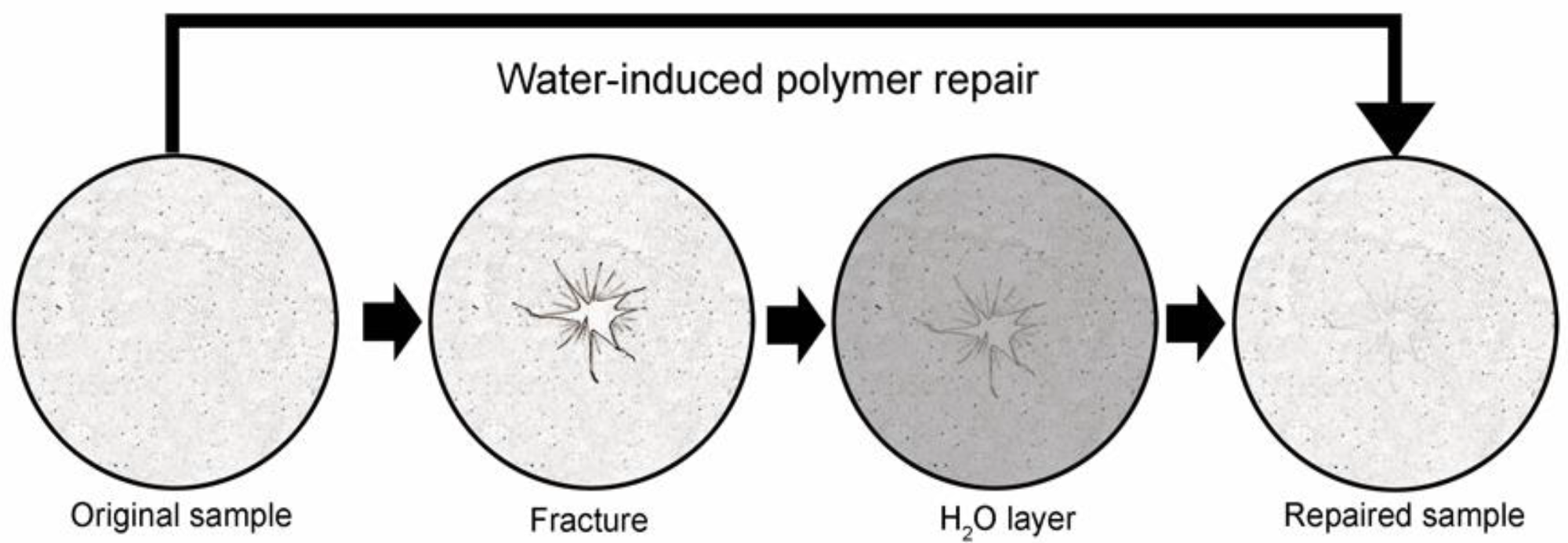
Schematic of water-induced repair of fractured pectin films. The glass phase pectin film is fractured using a controlled uniaxial load with a 5 mm stainless steel probe (2 mm/s). The fractured film is placed on a thin layer of distilled water (1.6 to 1.8 mm) for 8 h at 25 °C followed by optical and mechanical analysis of the repaired films. Image Credit: Pierce, A.F et al., Polymers
The Study
The authors have investigated the properties of pectin polymers. Specifically, they have studied how water can repair fractures in glass phase pectin films. Films were evaluated for resilience, cohesive strength, and optical properties. Additionally, the authors compared pectin biopolymers to alternative biopolymers made from oxidized cellulose, sodium hyaluronate, and nanocellulose fibers.
Citrus pectin was obtained from a commercial source for the study. Analysis of the polysaccharides revealed that they were composed mainly of galacturonic acid residues, with rhamnose, galactose, and arabinose. They also possessed 78-86% homogalacturonan and an RGI content of 16-26%. The pectin powder used in the research was stored in environmentally controlled conditions of low humidity and 25oC temperature. Pectin was dissolved in water and then poured into shaped molds for curing and further study.
The authors used several techniques to characterize the prepared pectin biopolymers. Fracture mechanics were studied by subjecting biopolymers to a controlled uniaxial load. The researchers recorded the fracture force, distance, and time.
Resilience was measured using a TA-XT plus, with a probe used to apply force and then retract at the same speed, with resilience defined as the ratio of the compressed area during compression and withdrawal. Scanning electron microscopy was used to image the pectin films after coating them with gold in an argon atmosphere. Additionally, statistical analysis was performed in the study.
The prepared pectin biopolymer films displayed near-complete water-induced self-repair. The films were incubated over a period of eight hours at room temperature and under ambient humidity. The process did not involve mixing or agitation. The mechanical strength of the repaired film was tested further by fracturing it for a second time, which demonstrated a decrease in the film’s burst strength, but the film was still significantly stronger in comparison to the other biopolymers in the research.
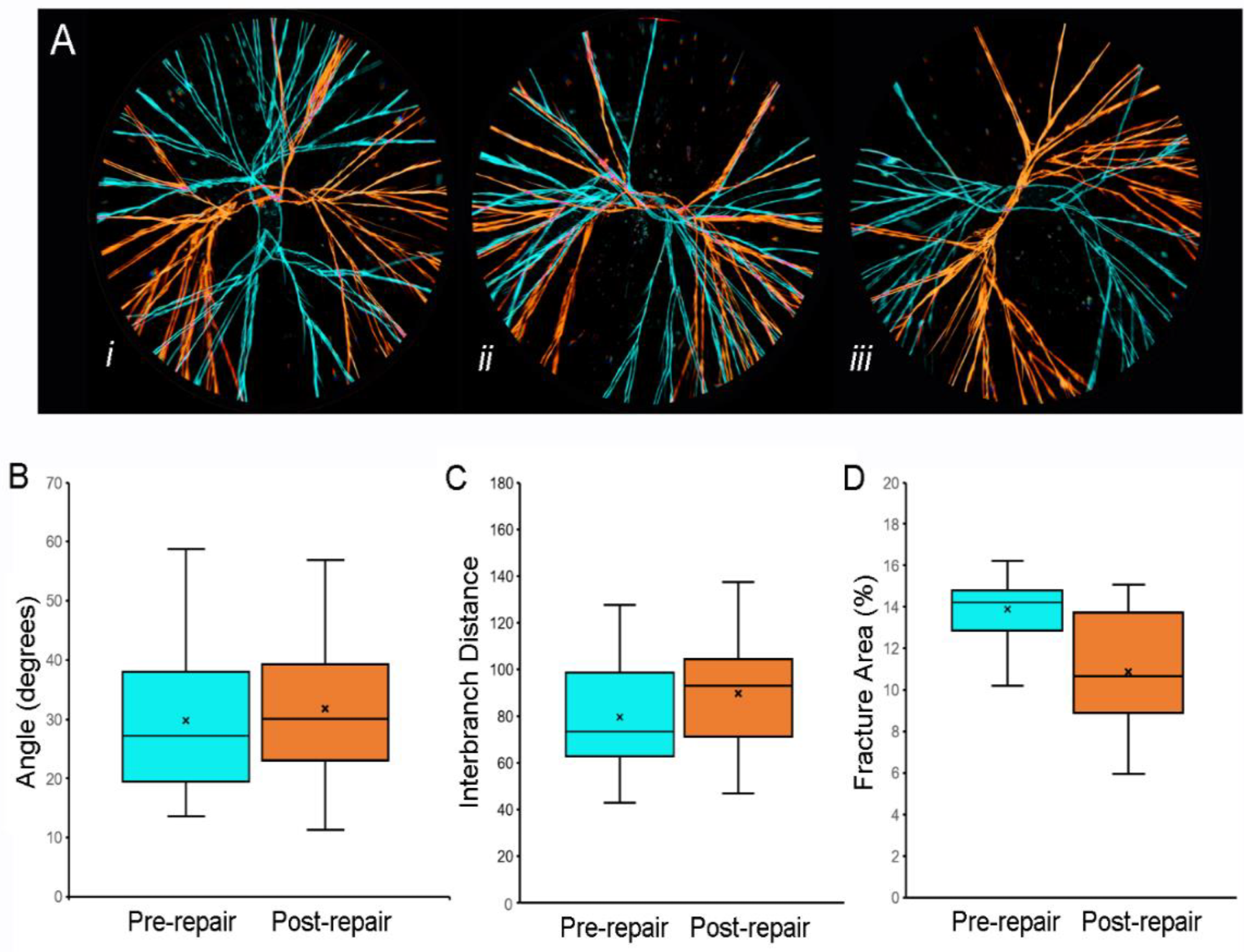
Fracture patterns of pectin films before and after water-induced repair. (A) Representative images of fracture patterns obtained before (blue) and after (orange) water-induced repair were thresholded and superimposed using the MetaMorph Stack Arithmetic Logical XOR function. Original orientation of the pectin films was maintained for comparison. The similar orientation of the fracture patterns in (Aii) was the exception; in most films, the fracture patterns appeared to be randomly oriented (e.g., (Ai) and (Aiii)). Morphometry of the fracture patterns demonstrated no significant difference in fracture angles (B), p > 0.05 or interbranch distance (C), p > 0.05. The total area of the pattern was slightly lower in the post-repair compared to the pre-repair fracture patterns (C), p < 0.05). In (B–D, the box spans the interquartile range with the median marked with an X and the whiskers defining the data range. A minimum of 103 features were measured for each parameter. Image Credit: Pierce, A.F et al., Polymers
The ability of the repaired films to withstand elastic deformation was also evaluated in the study. This was assessed by testing the resilience of the repaired films. The films were deformed by 1mm using a probe, and both the deformation force and release of stored energy were measured. The results of this test demonstrated that there was no significant loss in resilience in the water-repaired pectin films, with the resilience notably higher than the other polymers tested in the study.
In conclusion, the authors have demonstrated that pectin biopolymer films possess a notable degree of structural repair and retention of resilience to elastic deformation. This process is induced by a thin layer of water, which means that there is no need for complex techniques and harsh, potentially toxic, and environmentally damaging chemical solvents. Crucially, the reparative process demonstrated in the study is cell-free. The study has important implications for the commercial use of pectin biopolymers in the biomedical industry.
Further Reading
Pierce, A.F et al. (2022) Optical and Mechanical Properties of Self-Repairing Pectin Biopolymers [online] Polymers 14(7) 1345 | mdpi.com. Available at: https://www.mdpi.com/2073-4360/14/7/1345
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.