The function of new 2D materials and multilayer materials in the formulation of a corrosion prevention method has been evaluated by researchers in the journal Sustainability.

Study: Emerging Layered Materials and Their Applications in the Corrosion Protection of Metals and Alloys. Image Credit: Oasishifi/Shutterstock.com
Utilization of Metals and Alloys
Metals and alloys are crucial in modern life, and their qualities allow them to be employed in a broad array of applications. Aluminum and titanium, for example, are inexpensive and have applicability in the aviation industry, whereas copper or electroplated copper is commonly used in electrical applications, and steels are utilized in building and transportation.
Almost all metals are utilized as alloys or mixes of multiple elements since their qualities are better than those of pure metals. Alloying is done for a variety of purposes, the most common of which is to reinforce tensile characteristics, improve resistance to corrosion, or lower costs.
Corrosion Process in Metallic Alloys
Despite innovations in metal and alloy fabrication that have resulted in improved efficiency, however, metals and alloys are susceptible to corrosion. When a pure metal or alloy comes into the interface with its environment, it undergoes a continuous phenomenon that transforms it into its thermochemically preferred oxide, hydroxide, carbonate, or sulfide. As a result, the material gradually deteriorates and degrades, which can ultimately result in the loss of functionality, severe damage, or even environmental degradation due to the discharge of dangerous metal ions.
Additional metals or alloys are frequently used to replace broken components, which has issues for ecology due to over-utilization of environmental assets. Because sustainability is an important factor in today's climate, novel corrosion-protection measures are necessary.
The Corrosion Process
Two half-cell reactions can be used to characterize the corrosion process. The oxidation half process correlates to the transformation of metal to metallic ions, whereas the associated reduction half-reaction might entail either the reduction of absorbed O2 or the reduction of H+ (aq.), which becomes the preferred process under acidic circumstances.
As these processes progress, several types of rusting arise, such as generalized disintegration or localized rusting such as blistering, crevice, intergranular, or galvanic corrosion. To safeguard metals from corrosion, the flow of electrons must be slowed or stopped by limiting the oxidizing, reduction, or both halves of the process.
Importance of 2-D materials
Since the development of graphene nanoparticles, a slew of new 2D materials have been discovered and these substances, with their interesting features, are finding uses in a variety of fields including energy and sensing as well as the biomedical industry. It is no wonder that they are also becoming popular as corrosion-resistant compounds for metals and alloys. Graphene is one of the most commonly discussed of the different 2D substances that have drawn interest in the creation of corrosive prevention techniques. However, graphene's long-term corrosion-protective ability is not always acceptable.
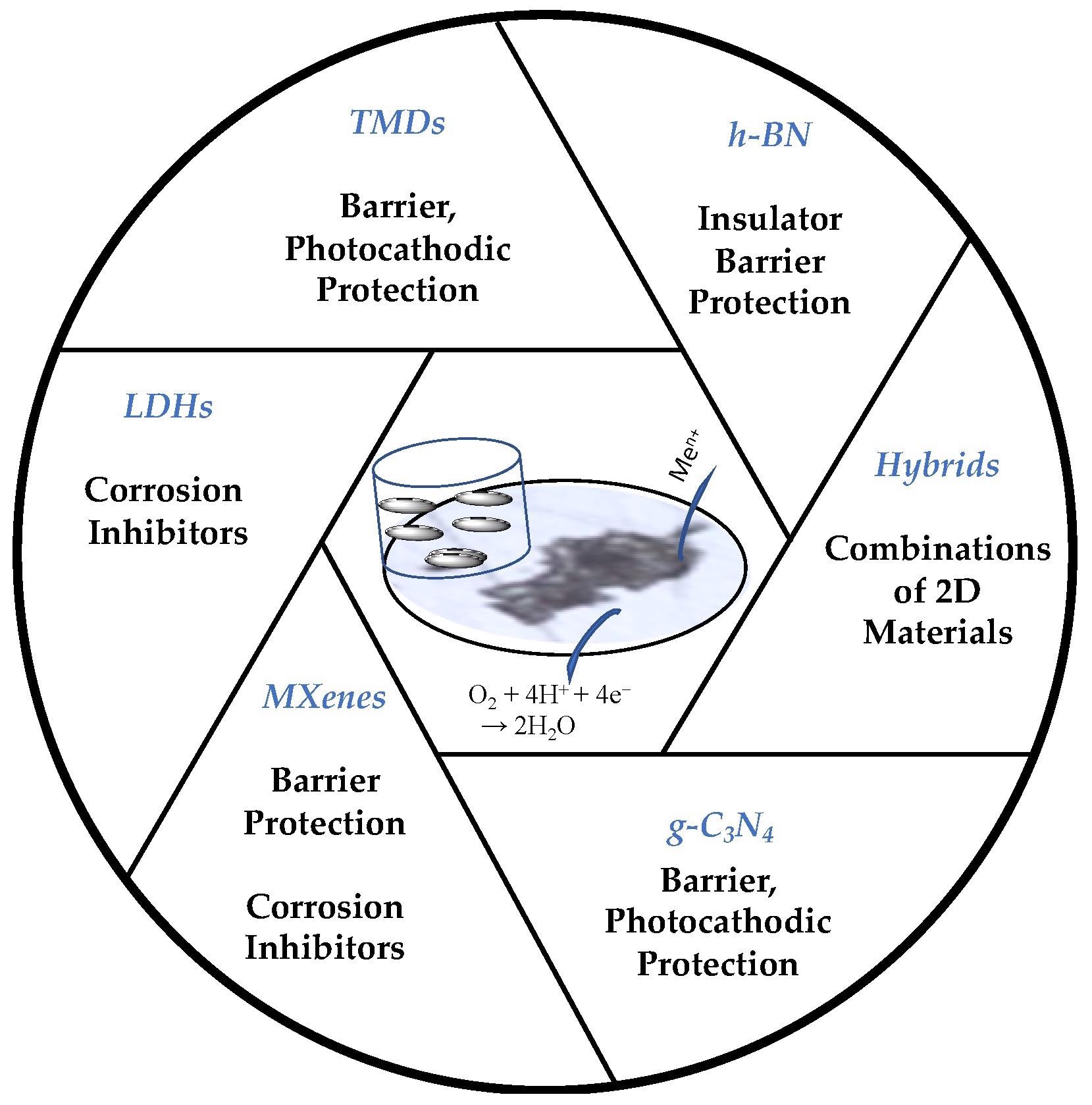
Schematic of the 2D materials and their primary roles in corrosion protection. Sukanya, R. et. al, Sustainability
Other 2D Layered Materials
MXenes are a fascinating class of 2D materials made out of metal carbides or nitrides. MXenes are a collection of layered transition metal carbides and nitrides that have been carefully peeled from their basic MAX structures. The precursor of MXene, MAX material phase, has the chemical formula Mn+1AXn (n = 1 to 4), where M is an initial transition metallic element, A is an element from groups 13-15 of the periodic table, and X is the carbide or nitride group. Others include Graphitic Carbon Nitride (g-C3N4) and h-BN substances with properties closely resembling graphene.
Corrosion Protective Coatings
Corrosion-protective coatings have received a lot of interest in a range of applications because they act as a barrier between the surroundings and the underlying metal or alloy. The optimal covering should be resistant to humidity and corrosive substances and have long-term durability without breaking or debonding, which are the major failure causes.
Layered and 2D materials are particularly appealing in this respect because they can improve barrier protection by preventing the flow of oxygen, water, and highly corrosive ions such as chloride anions. In principle, the impermeable quality of the 2D sheets may be used to not only delay diffusion but also to plug gaps in the coatings, prolonging their protective time.
Future Perspective
Many previous corrosion-protection methods depended on chromates and volatile organic material, both of which are no longer ecologically acceptable. While structural materials have made great advances in the case of mechanical qualities, their abilities to deliver and tolerate rusting remains a special problem. While 2D graphene has been widely investigated, there is now agreement that the conducting features of graphene promote corrosion problems with the substrates and can actually enhance the corrosion process.
While obstacles exist, it is obvious that these 2D materials will perform an important role in the future generation of protective and corrosion-resisting coatings.
Further Readings
Sukanya, R. et. al. Emerging Layered Materials and Their Applications in the Corrosion Protection of Metals and Alloys. Sustainability 2022, 14(7). 4079. Available at : https://www.mdpi.com/2071-1050/14/7/4079
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.