Professor Guowen Meng’s group from the Chinese Academy of Sciences’ (CAS) Hefei Institutes of Physical Science (HFIPS) has developed an effective electrocatalytic material to convert nitrate in water to nitrogen gas. The study was published in Chemical Communications.
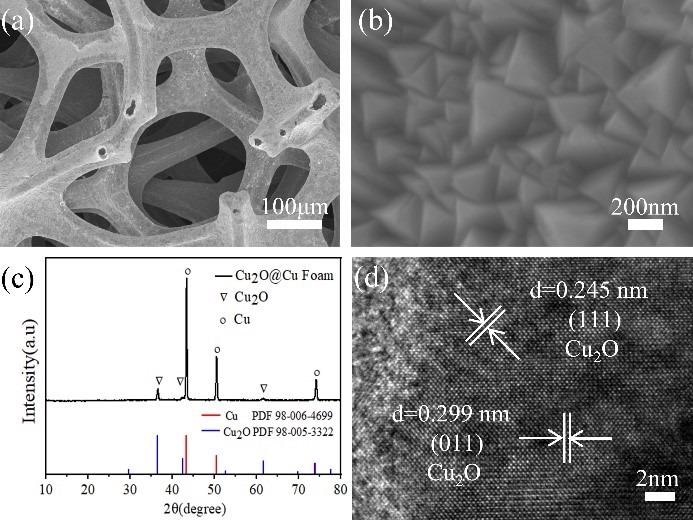 (a-b) SEM images and (c) XRD pattern of the electrodeposited Cu2O thin films on frame of Cu foam; (d) HRTEM image of the as-prepared Cu2O thin films. Image Credit: Haibin Tang.
(a-b) SEM images and (c) XRD pattern of the electrodeposited Cu2O thin films on frame of Cu foam; (d) HRTEM image of the as-prepared Cu2O thin films. Image Credit: Haibin Tang.
Associate Professor Haibin Tang, who was in charge of this research, said “This material is a success in improving the selectivity of electrocatalytic reduction of nitrate for N2 in water.”
Nitrate, a hazardous water pollutant, is known to cause eutrophication, blooming and other environmental and ecological issues.
The electrocatalytic reduction process stands out among nitrate removal methods and technologies because it can preferentially transform nitrate (NO3-) into ammonium (NH4+) or nitrogen gas (N2), effectively lowering the total nitrogen concentration in water and showing tremendous application potential for restoring eutrophicated water bodies.
However, despite having preferential selectivity and efficiency towards ammonia/ammonium, the materials previously described are not suited for use in actual aquatic conditions, such as lakes and rivers.
The electrochemical deposition was used to create a (111) selectively oriented copper oxide (Cu2O) coating on the surface of a porous copper foam framework (Cu2O@CF) in this research. The composite construction can readily be used as a cathode for electrocatalytic nitrate reduction thanks to the conductive porous copper foam frame. The catalytic reduction of nitrate to N2 was greatly improved, according to the findings.
In alkaline solution, the removal rate of nitrate is 93% and the selectivity of N2 is 99%; and in neutral solution, the removal rate of nitrate was 94.3% and the selectivity of N2 is 49.2%.
Haibin Tang, Associate Professor, Chinese Academy of Sciences
Electrocatalytic tests were performed utilizing lake water from a local reservoir to validate the potential influence of other cations and anions in actual water.
The removal rate reached as high as 91.1% for nitrate and the selectivity for N2 rose up to 64.2%, both were higher than what we had expected.
Haibin Tang, Associate Professor, Chinese Academy of Sciences
The cathode material used in this study is simple to make and has a high selectivity for nitrogen gas, indicating that Cu2O could be used in the electrocatalytic nitrate reduction to nitrogen gas.
This research can be used to develop more stable and efficient electrocatalytic materials for removing nitrogen from water, which is beneficial for water treatment and environmental protection.
The study was funded by the Chinese National Natural Science Foundation, the Natural Science Foundation of Anhui Province, and the Chinese Academy of Sciences’ Key Research Program of Frontier Sciences.
Journal Reference:
Zhao, Q., et al. (2022) Efficient electrocatalytic reduction of nitrate to nitrogen gas by a cubic Cu2O film with predominant (111) orientation. Chemical Communications. doi.org/10.1039/D1CC07299D.