Zinc-ion batteries have been proposed to overcome the safety and cost concerns of conventional battery technologies. Writing in the journal ACS Applied Energy Materials, a team of scientists from China has investigated using chelate electrolyte additives to help realize the development of these next-generation energy storage devices.

Study: Toward Stable Zn-ion Batteries: Use of a Chelate Electrolyte Additive for Uniform Zinc Deposition. Image Credit: Illus_man/Shutterstock.com
Zn-ion Batteries
Lithium-ion batteries are widely utilized in multiple industries due to their superior energy efficiency, longer lifespan, fast charging times, and compact, lightweight construction. However, there are significant safety and cost concerns with these devices. They also have issues with sustainability, abundance, and environmental damage caused by the extraction of critical resources.
Zn-ion batteries have emerged in recent years as a promising alternative to conventional lithium-ion batteries to overcome these critical issues. However, Zn-ion batteries have problems with low coulombic efficiency and poor cycling performance, which limits their widespread application. This is caused by the zinc anode, which is prone to failure.
Zn2+ accumulation can occur on interfacial nucleation sites. A heterogenous interfacial electrical field can be formed caused by the generation of zinc dendrites during the plating process. Short-circuiting can occur due to the continuous deposition of metallic zinc and the formation of protuberances. Dendrite formation can trigger side reactions, leading to a zinc passivation layer in the devices.
Researchers have attempted to develop high-performance zinc anodes by balancing the interfacial electrical field and regulating the migration of zinc ions in devices. Strategies explored include surface modifications, tailoring the structure of devices, and optimizing electrolytes. The use of additives in electroplating baths has been investigated to regulate the deposition kinetics.
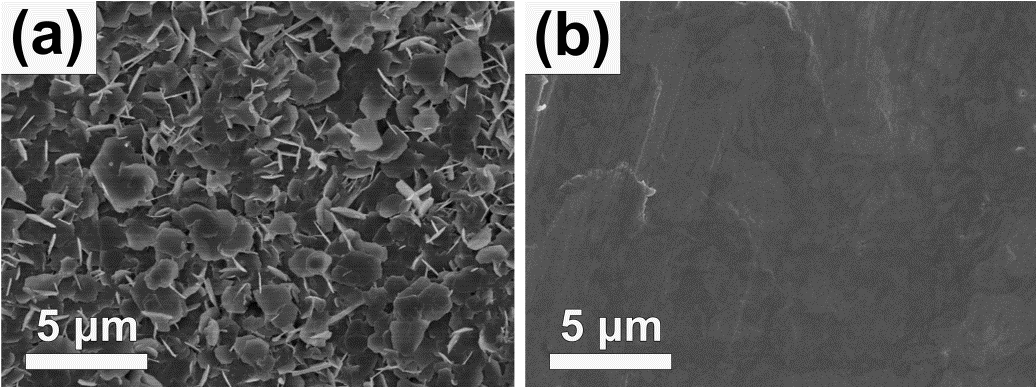
SEM images of Zn anodes after initial striping for 1 h in (a) blank ZSO and (b) EDTA added electrolyte at a current density of 1 mA cm-2. Image Credit: Xie, K et al., ACS Applied Energy Materials
The Research
The authors behind the study have investigated the use of EDTA as a chelating agent. This chemical is used as an electrolyte additive to fabricate dense, flat metal or alloy deposits. Zinc possesses an affinity for EDTA, and due to the chelation with Zn2+ ions, increased active sites for initial nucleation are produced. Thus, during the initial plating stage of Zn-ion battery fabrication, a smaller grain size is facilitated. This leads to a compact, flat plating layer during subsequent plating without a layer of “dead” zinc.
This method produces Zn-ion batteries with improved cycling stability. Furthermore, the coordination environment of Zn2+ ions is changed by the use of EDTA as a chelating agent, suppressing the instance of side reactions and reducing the risk of short-circuits. Using EDTA improves the cycling performance of both symmetric and full cells.
Materials for the study were prepared by dissolving EDTA in a 2 m zinc sulfate solution. The solution was kept at 70 oC and continuously stirred until the EDTA had dissolved completely. The structure of the resulting composition was analyzed using X-ray diffraction, X-ray photoelectron spectroscopy, and energy-dispersive X-ray spectroscopy. Surface roughness was categorized using atomic force microscopy. A contact angle goniometer was utilized to measure the contact angle.
The novel EDTA-zinc sulfate composition was used to construct batteries that possessed a coin cell configuration. Sample devices without an EDTA electrolyte were prepared for comparison, and glass fiber was used as a separator. V2O5 was used as a cathode in the devices. Electrochemical performance, EIS, and cyclic voltammetry tests were conducted on the batteries.
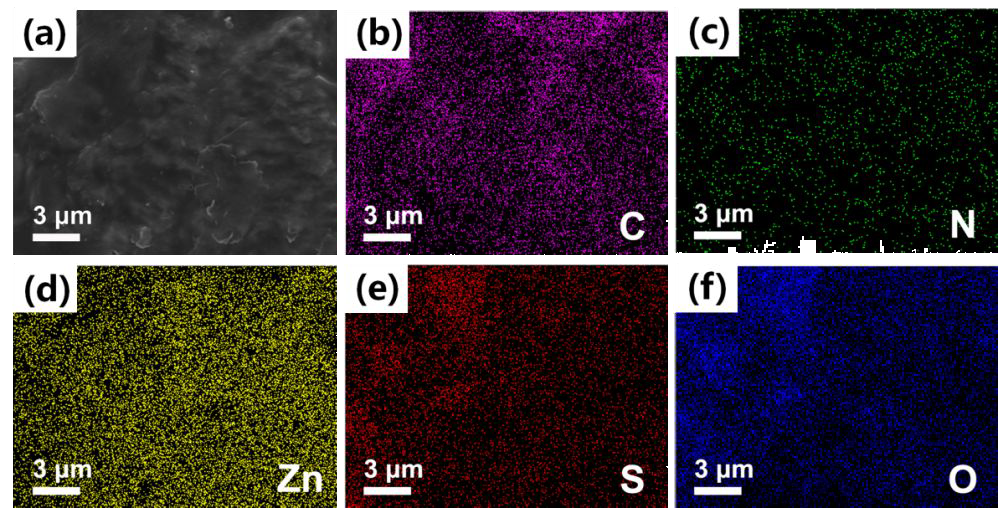
(a) SEM image and corresponding EDS mapping images of (b) C, (c) N, (d) Zn, (e) S and (f) O elements in the Zn anode after cycled for 500 h in EDTA added electrolyte (1 mA cm-2 ~ 1 mAh cm-2). Image Credit: Xie, K et al., ACS Applied Energy Materials
Results of the Study
After ten minutes of deposition in pure zinc oxide electrolyte, unevenly distributed protrusions started to appear on the zinc anodes. Upon further cycling, these protuberances formed dendrites, and the anode suffered from a proliferation of cracks, indicating an inhomogeneous metallic zinc deposition in the devices.
Conversely, with the use of the EDTA electrolyte, a smooth edge was produced on the zinc anodes during the entire deposition process, free of dendrites. Analysis of surface morphology indicated that EDTA has a refining effect for Zn deposition and helps to avoid the formation of dendrites during repeated cycling. SEM was used to investigate the effect of EDTA on zinc stripping, and again a smooth surface was observed, further indicating that EDTA facilitates dendrite-free zinc plating.
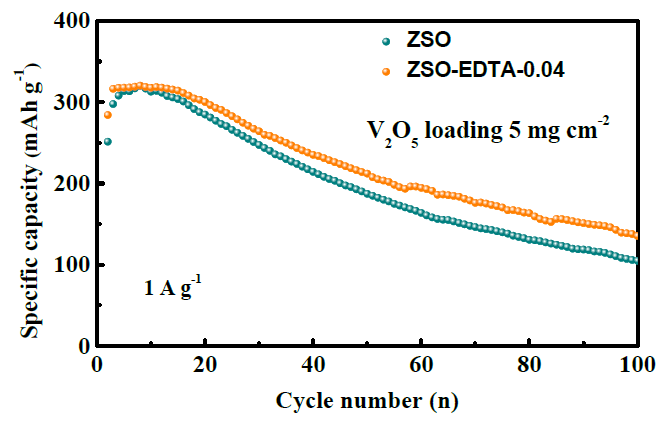
Long-term cycling performance of Zn-V2O5 full cells in high mass loading of 5.0 mg cm-2. Image Credit: Xie, K et al., ACS Applied Energy Materials
Superior cycling ability in the EDTA electrolyte cells was observed by the authors, with a high reversible capacity of the cells after 500 cycles. Good cycling efficiency was observed after 100 cycles.
The research demonstrated a simple and effective strategy for developing Zn-ion batteries with superior performance and cycling efficiency, which overcomes the problems with conventional lithium-ion batteries as well as addresses the challenges in developing next-generation Zn-ion batteries. This study provides a road toward developing safer, more sustainable energy storage devices for future use.
Further Reading
Xie, K et al. (2022) Toward Stable Zn-ion Batteries: Use of a Chelate Electrolyte Additive for Uniform Zinc Deposition [online] ACS Appl. Energ. Mater. | pubs.acs.org. Available at: https://pubs.acs.org/doi/10.1021/acsaem.1c03558
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.