Reviewed by Alex SmithApr 26 2022
The increasingly severe environmental issues and energy crisis have resulted in the quick development of renewable energies and their higher proportion in the energy-supply structure.
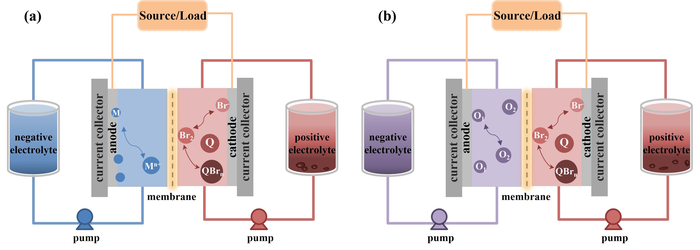 Schematic diagrams of Br-FBs with different negative redox couples: (a) metallic redox couple and (b) non-metallic redox couple or organic molecular redox couple. Image Credit: Division of energy storage, Dalian Institute of Chemical Physics.
Schematic diagrams of Br-FBs with different negative redox couples: (a) metallic redox couple and (b) non-metallic redox couple or organic molecular redox couple. Image Credit: Division of energy storage, Dalian Institute of Chemical Physics.
The research and development of large-scale energy storage technologies are considered essential to realize the extensive use of renewable energies because they currently have broken, uncontrollable and unstable characteristics.
Bromine-based flow batteries (Br-FBs) are considered to be one of the most hopeful energy storage technologies with appealing benefits of low cost, extensive potential window and a long cycle life.
But, Br-FBs suffer from the torpid kinetics of Br2/Br- redox couple and severe self-discharge, impeding the additional commercialization and industrialization of Br-FBs. Electrodes, one of the critical components in a Br-FB, provide the reaction sites for redox couples and has a considerable impact on the Br-FBs’ performance.
In a study, recently reported in the journal Energy Material Advances, Xianfeng Li et al. from the Dalian Institute of Chemical Physics, the researchers outlined the properties, benefits and drawbacks of various types of cathode materials for Br-FBs and outlined appropriate modification methods.
This offers extensive and available instructions to come up with high-performance cathodes for long-lifespan and high-power density Br-FBs.
Initially, the authors made a comparison of the commonly utilized cathode materials for Br-FBs. These were categorized as metal-based compounds, like Pt, and carbon-based materials, such as graphite felts (GFs) and carbon felts (CFs).
Regarding metal-based materials, besides a few valuable metals and oxides, the majority of the metals and metal oxides tend to be unstable in the existence of highly corrosive bromine. Until now, metal-based cathode materials available at present for Br-FBs are Pt, ZrOx, TiN, TiOx, WOx, etc.
Carbon-based materials have been extensively utilized as cathode materials of Br-FBs, as their electrochemical activity could be improved by controlling the surface properties and internal structure.
Currently, porous carbon fiber-based materials, particularly GFs and CFs, are the most generally utilized cathodes in Br-FBs as a result of their cost-effectiveness, excellent corrosion resistance, good electronic conductivity and controllable surface properties.
Later, the authors focused on the alteration methods of GFs and CFs, such as surface modification and surface treatment. The surface treatment is to build porous structures intrinsically on the surface of the electrode by oxidizing etching. This can increase the particular surface area and initiate catalytic functional groups.
But surface treatment is often utilized as a pretreatment technique due to uncontrollable distribution of pores and worsened mechanical properties. Different from surface treatment, surface modification is to help increase the electrochemical activity and hydrophilicity of the electrodes by initiating active materials.
Depending on the properties of introduced active materials, surface modifications could be categorized as the nonmetallic element modification, metallic element modification, and the structure decoration. The costs of metallic element modification are very high and the modifier is not quite stable in Br-FBs.
While for the nonmetallic element modification, even though element doping adds up to the enhanced activity and good wettability, the altered electrodes tolerate nonuniform element distribution and poor mechanical stability.
Remarkably, the generally utilized CF/GF-based electrode materials possess an adjustable structure and good electronic conductivity. Consequently, controlling the structures of the electrode by the structure decoration technique seems to be beneficial and has been generally utilized to improve the performance of cathodes of Br-FBs.
Eventually, the authors outlined the future development directions of Br-FBs cathodes. The development of cathode materials exhibiting high stability, activity and bromine fixing or retention ability is considered essential to help tackle the technical bottlenecks of severe self-discharge and the low power density of Br-FBs.
Hence, there still pertains a great potential for the development of improved cathode materials of Br-FBs and additional research on the mechanisms of Br2/Br- reactions. Listed below are the additional development directions:
(i). To constantly develop sophisticated Br-FBs electrodes possessing high activity and bromine retention capacity by improving their chemical surfaces and enhancing their physical structures.
(ii). The reaction mechanisms on the electrode are not evident and are in need of in-depth investigations.
(iii) To enhance the bromine fixing or retention capacity of electrodes with the help of structural design, namely, to hinder the diffusion and migration of bromine species to the negative side.
This study was financially supported by the National Natural Science Foundation of China (Grant No. 21206158), Key Project of Frontier Science, CAS (QYZDBSSW-JSC032), DICP funding (DICP I202026 and DICP I201928), and Liaoning Natural Science Foundation.
Journal Reference:
Tang, L., et al. (2022) Progress and Perspective of the Cathode Materials towards Bromine-Based Flow Batteries. Energy Material Advances. doi.org/10.34133/2022/9850712.