Metal ion pollution is a key challenge in environmental remediation currently, requiring powerful and reliable detection methods to monitor levels of ions in the environment. A team of researchers from Egypt has presented a novel approach to creating calcium sensors: incorporating DNA. Their work has appeared in the journal Polymers.

Study: Polymeric Electrochemical Sensor for Calcium Based on DNA. Image Credit: Peshkova/Shutterstock.com
Detecting Metal Ions in the Environment
The rapid growth in urbanization and industry has led to vast amounts of organic and inorganic pollutants entering the environment. Amongst these pollutants, metal ions are particularly problematic. Their prevalence and non-degradability lead to proliferation in ecosystems and a deleterious effect on the food chain due to their bioavailability.
Due to these issues, reliable and effective ion sensors have become a research focus recently. Qualitative and quantitative metal ion detection is a key concern for scientists working in the fields of materials science and environmental remediation.
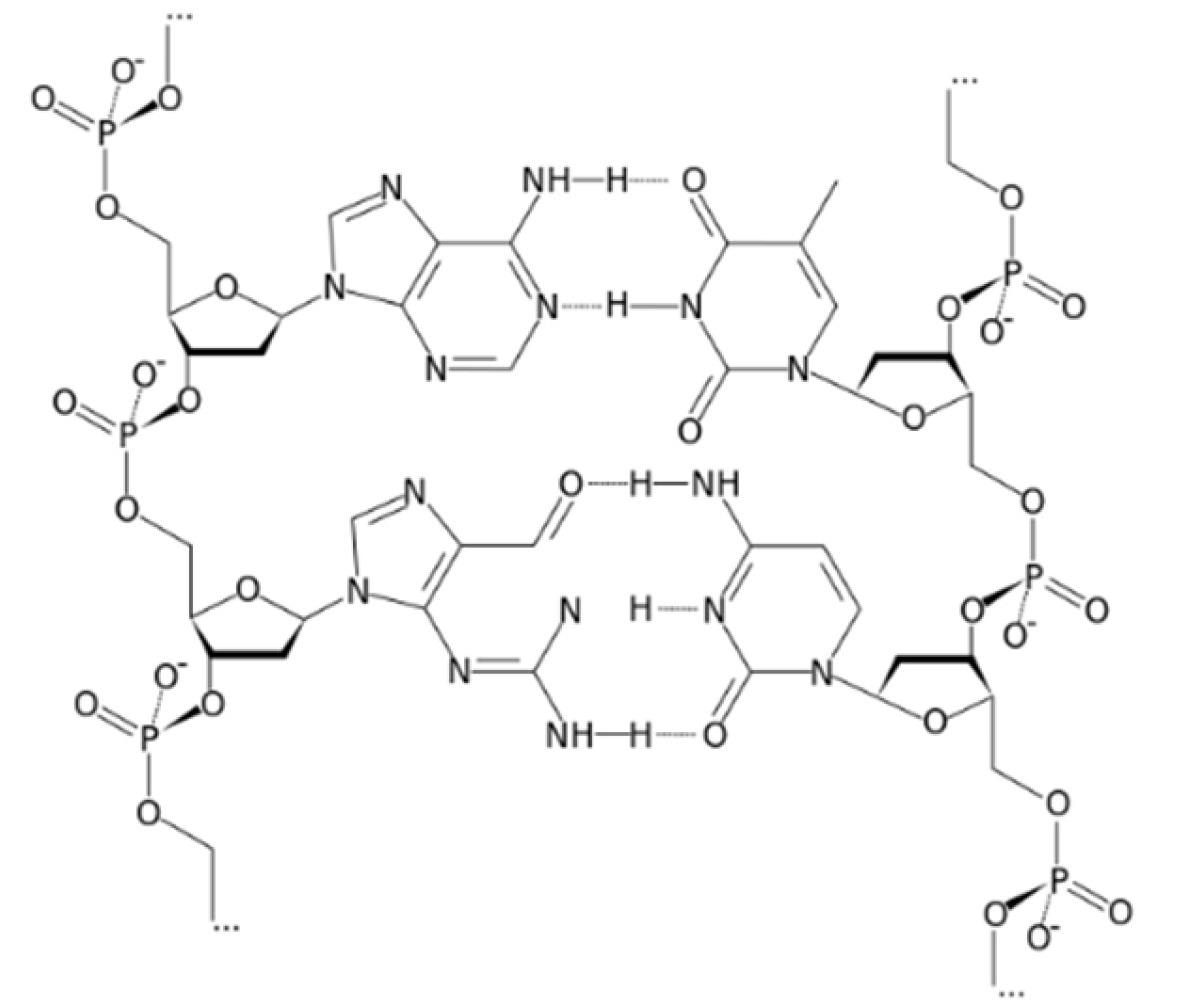
Structure of deoxyribonucleic acid (DNA). Image Credit: Zareh, M.M et al., Polymers
Strategies for Detecting Ca2+ Ions
Ca2+ ions are released by several industries and from numerous commercial products. Determining the levels of calcium ions is important for areas such as food assessment, medical diagnosis, and domestic and industrial water hardness control. Calcium is a chief component of the skeletal system in animals, and is important for bone and teeth development, along with other biological functions.
Several strategies have been developed in recent years to detect and determine the levels of calcium ions in the environment. Methods such as NMR, molecular fluorescent chelators, and flame atomic absorption spectroscopy have been employed by researchers for this purpose.
Other techniques developed by researchers include paper-based microfluidic devices and wearable electrochemical sensors for continuous monitoring of calcium ion levels. An interesting development is the introduction of genetically encoded sensors based on protein engineering principles. However, current strategies have a fairly high limit of detection.
Current technologies, whilst accurate, are not suitable for the detection of Ca 2+ ions in environmental samples. However, ion sensors are extremely valuable for detecting calcium ions when there are a large number of samples, due to their low cost, straightforward operation, rapid detection, and online monitoring capabilities.
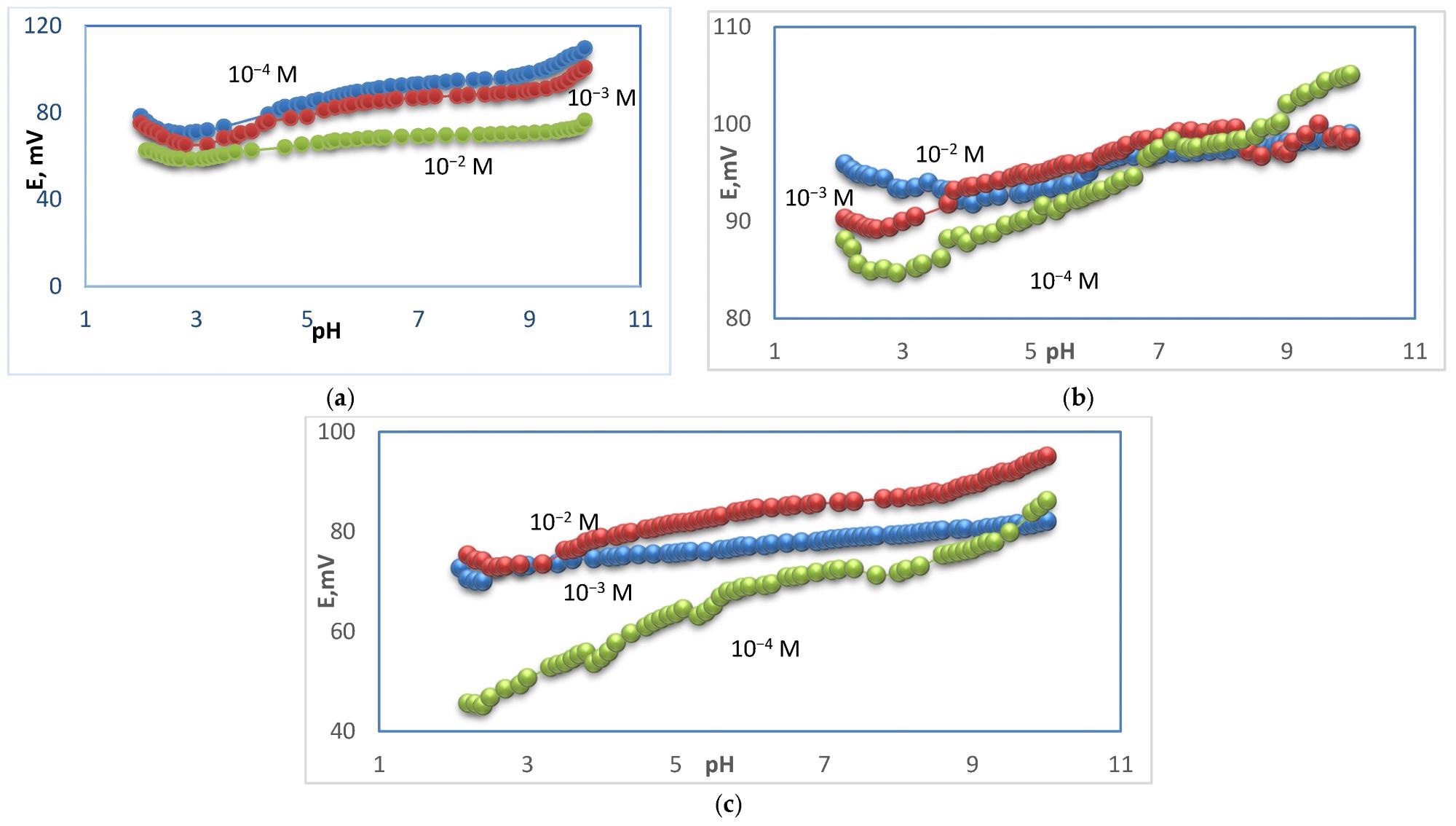 pH–effect of Ca–electrode for 10−2, 10−3, 10−4 M Ca2+ solutions for electrode types I (a), type II (b), and type III (c). Image Credit: Zareh, M.M et al., Polymers
pH–effect of Ca–electrode for 10−2, 10−3, 10−4 M Ca2+ solutions for electrode types I (a), type II (b), and type III (c). Image Credit: Zareh, M.M et al., Polymers
Optical and Electrochemical Sensor Platforms
Optical and electrochemical sensors can overcome the drawbacks of conventional platforms. Several ion-selective electrodes have been developed in recent research for this purpose. One study has developed a calcium ion-selective electrode that utilizes a surface-modified ionophore based on zeolite, which is highly efficient. This sensor displayed high selectivity and sensitivity over monovalent cations. When tested on real samples, the sensor performed well.
The development of calcium ion sensors using nano-sized imprinted polymers as ionophores have been reported in recent years, as well as devices that utilize rGO-coated black phosphorous as a composite mediator layer. Devices have displayed good lifetime performance, a limit of detection, and E.M.F value reproducibility. Fine tip Ca2+ selective electrodes have been evaluated recently, with favorable slope and limit of detection.
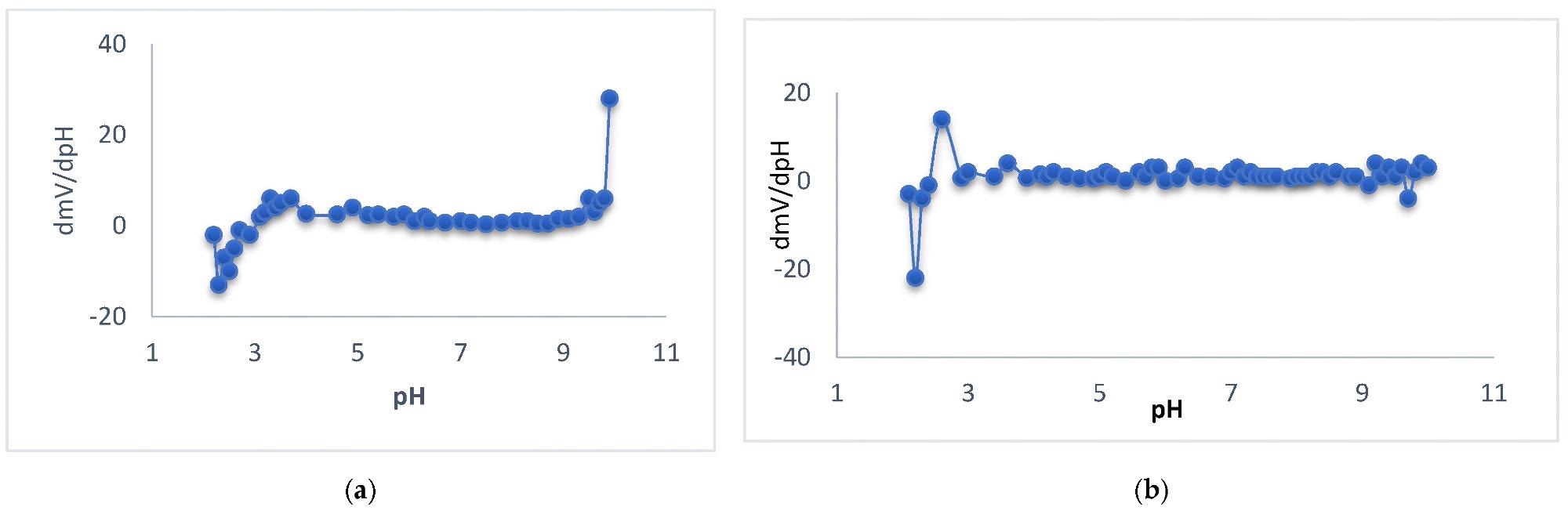
Representation of ∂mV/∂pH against pH value of Ca sensor for 10−2 M Ca2+ solutions for the sensors type I (a) and type III (b). Image Credit: Zareh, M.M et al., Polymers
The Paper
An innovative approach has been taken by the researchers behind the current paper in the journal Polymers. In the proposed system, DNA is used as the ionophore in a novel electrode for calcium ion detection. The device takes advantage of numerous polar sites which exist naturally on DNA and facilitates the attachment of cationic species. This DNA-based ionophore is different than conventional ionophores as it can be regarded as a polyion.
Three electrode plasticizers were used: nitrophenyl octyl ether, diethyl-phthalate, and dioctyl Phthalate. The membrane matrix used was PVC. Membrane components were dissolved using tetrahydrofuran. Analytical-grade chloride and sulfate reagents were used to perform selectivity studies. Solutions of calcium chloride with different concentrations were used in the research.
The novel DNA-based electrode sensor was prepared, and the membrane studied was composed of DNA:DOP:PVC. 2 mg of DNA, 60 mg PVC, and 120 mg of the solvent mediators were used, and dissolved components were poured into a 3 cm diameter petri dish and allowed to dry under room temperature conditions. 7 mm diameter samples were used to prepare the electrode and attached to a sensor. This sensor was then soaked in a 0.01 M CaCl2 solution for 24 h and was then characterized.
Amongst the three plasticizers used in preparing the electrodes, diethyl-phthalate performed the best. The worst performance was observed using nitrophenyl octyl ether, which agrees with previous studies. The sensor displayed sufficient performance for detecting calcium ions in real samples.
The present study has demonstrated the suitability of DNA as an extremely sensitive and selective calcium ion-detecting ionophore. The development of this novel environmental calcium ion sensor presents an innovative solution to the issue of detecting metal ion pollution. Finally, the authors have stated that their work should encourage future studies on other environmentally critical cationic metal species.
Further Reading
Zareh, M.M et al. (2022) Polymeric Electrochemical Sensor for Calcium Based on DNA Polymers 14(9) 1896 [online] mdpi.com. Available at: https://www.mdpi.com/2073-4360/14/9/1896
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.