A paper recently published in the journal ACS Applied Energy Materials demonstrated the effectiveness of magnesium/boron (Mg/B) solid solutions synthesized using the dry powder-based technique for energetic applications.

Study: Synthesis and Characterization of Magnesium/Boron Solid Solutions for Energetic Applications. Image Credit: magnetix/Shutterstock.com
Background
Highly energetic materials such as metalloids and metals are often added to propellants and rocket fuels to produce more thrust and extract more energy in volume-limited propulsion systems. These materials are more suitable as fuel additives or fuel compared to liquid hydrocarbon fuels as they possess higher volumetric and gravimetric energy density, leading to reduced exhaust emissions and improved combustion efficiency.
Boron is the most suitable metal as a fuel additive and solid fuel due to its high volumetric and gravimetric densities of 140 kJ/mL and 58 kJ/g, respectively. Reactive metals such as aluminum (Al) and magnesium (Mg) have also been studied extensively for energetic applications due to their notably high gravimetric energy densities of 31 kJ/g and 25 kJ/g, respectively.
However, the existence of a native oxide layer on the B surface limits the efficiency of the metal as an energetic material. Although the oxide layer provides temporary passivation during storage, it acts as a diffusion barrier at the oxidizer/boron interface that blocks direct reaction between the metal and the oxidizer and inhibits ignition kinetics, leading to incomplete combustion. Thus, reducing the metal oxide layer thickness is necessary for improving the exothermicity and reactivity during B particle oxidation.
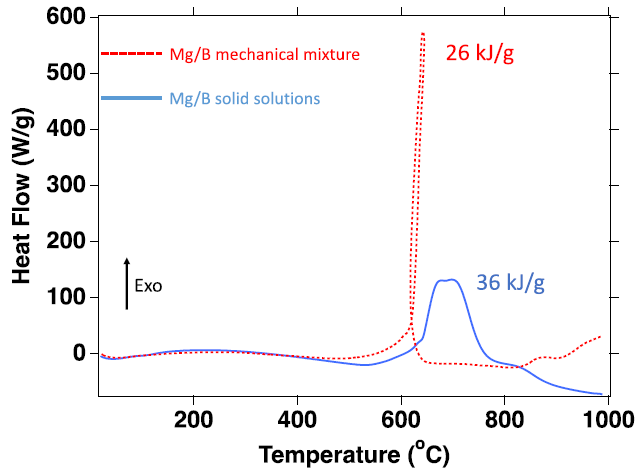
Comparison of DSC analysis of Mg/B mechanical mixture (red) and a Mg/B solid solution (blue). The mechanical mixture releases 26 kJ/g and the solid solution releases 36 kJ/g up to 1000 ℃, an increase of 38% in heat release. Image Credit: Jensen, D et al., ACS Applied Energy Materials
Catalytic dopants, such as Mg and Al, can be used to remove the native B oxide layer through redox reactions and reduce the ignition delay. These dopants can increase the amount of heat released during B particle oxidation by reducing the B oxide to elemental B. Additionally, the ignition of dopants increases the reaction interface temperatures, improving the B oxidation kinetics.
Due to its high energy density, Mg has been used successfully as a propellant additive in underwater and aerospace propulsion systems. The material possesses relatively low boiling and melting points and good combustion characteristics, which can facilitate ignition and complete oxidation of B.
The addition of Mg in the form of a compound with B, such as magnesium diboride (MgB2), can further improve the combustion efficiency by increasing the extent of oxidation. Additionally, metal borides such as MgB2 also have a prolonged shelf life owing to their high thermal stability.
The Study
In this study, researchers synthesized energetic B/Mg solid solutions using the self-propagating high-temperature synthesis (SHS) reaction method between Mg and B. Later, they compared the thermochemical behavior of B/Mg solid solutions with pure B particles to evaluate the effects of Mg addition on the oxidative stability of B at low temperatures and gravimetric heat release of B particles upon oxidation.
Submicrometer-sized Mg and B particles with high purity were used to fabricate the core-shell B/Mg structures, ensuring the high purity of the solid solutions. The SHS reaction was selected for the dry synthesis of the solid solutions due to the self-sustaining nature of the reaction upon initiation.
B and Mg powders were mixed into a glass vial using the three-step dry-mixing technique. Initially, the dry mixture was stirred using a micro-spatula and then sonicated at 25 oC for 15 min to disperse the aggregates. Subsequently, the as-prepared mixture was subjected to magnetic agitation to obtain a homogenous mixture.
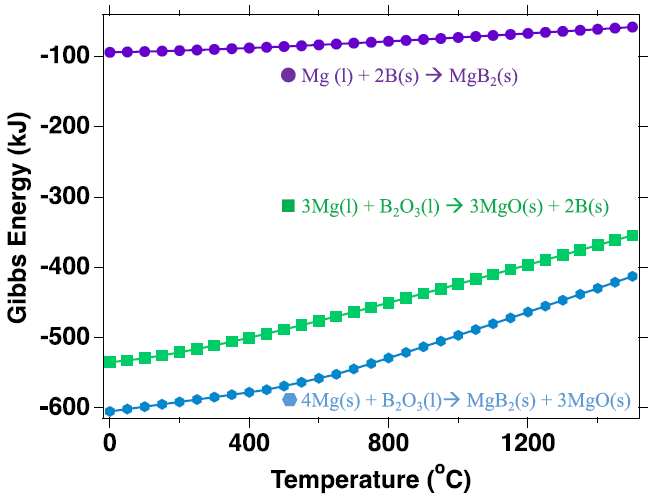
Gibbs energy as a function of temperature for different reactions between Mg, B and their respective oxides at 500 ℃. Thermodynamic analysis was conducted the HSC industrial thermodynamic software package. Image Credit: Jensen, D et al., ACS Applied Energy Materials
The obtained mixture was spread uniformly over a glass slide surface, and another glass slide was placed on the mixture. An Al foil was used to cover the sides of the mixture. The mixture was then pressed between the glass slides to promote close contact between B and Mg and reduce the particle exposure to ambient air to facilitate the effective diffusion of atoms during heating.
Subsequently, the glass slides were placed in a muffle furnace at 25 oC and heated at a rate of 30 °C/min to 500 °C. The isothermal conditions at 500 °C were maintained for 90 min, and then the heated sample was allowed to cool for three h. Thermal analysis experiments were performed on different synthesized samples to optimize the process parameters, such as synthesis time and temperature.
X-ray diffraction (XRD), dynamic light scattering (DLS), X-ray photoelectron spectroscopy (XPS), and high-angle annular dark-field scanning transmission electron microscopy energy dispersive spectroscopy (HAADF-STEM-EDS) were used to characterize the fabricated B/Mg solid solutions. Differential scanning calorimetry (DSC) and thermogravimetric analysis were performed to determine the weight change and heat flow on the samples from 20 to 1000 oC.
Observations
Energetic B/Mg solid solutions were synthesized successfully with an average particle size of 550 nm through the SHS reaction. The synthesis conditions maintained a maximum contact between B and Mg particles and minimized sintering of the synthesized samples.
A commensurate MgB2/MgO layer was formed around the B core due to the direct contact between the surface of B particles and liquid Mg. HAADF-STEM-EDS, XRD, and XPS observations confirmed the formation of an outer shell composed of MgB2, MgO, and Mg around the B core.
The core-shell structure facilitated passivation shell formation, exothermic redox reaction between the native B oxide layer and Mg, and exothermic oxidation of B, MgB2, and Mg. The exothermic heat release due to B combustion in the B/Mg solid solutions increased by 24% compared to the heat released by untreated B particles at temperatures up to 1000 °C. The oxidative stability of the B/Mg solid solutions was also increased compared to B and Mg under similar conditions.
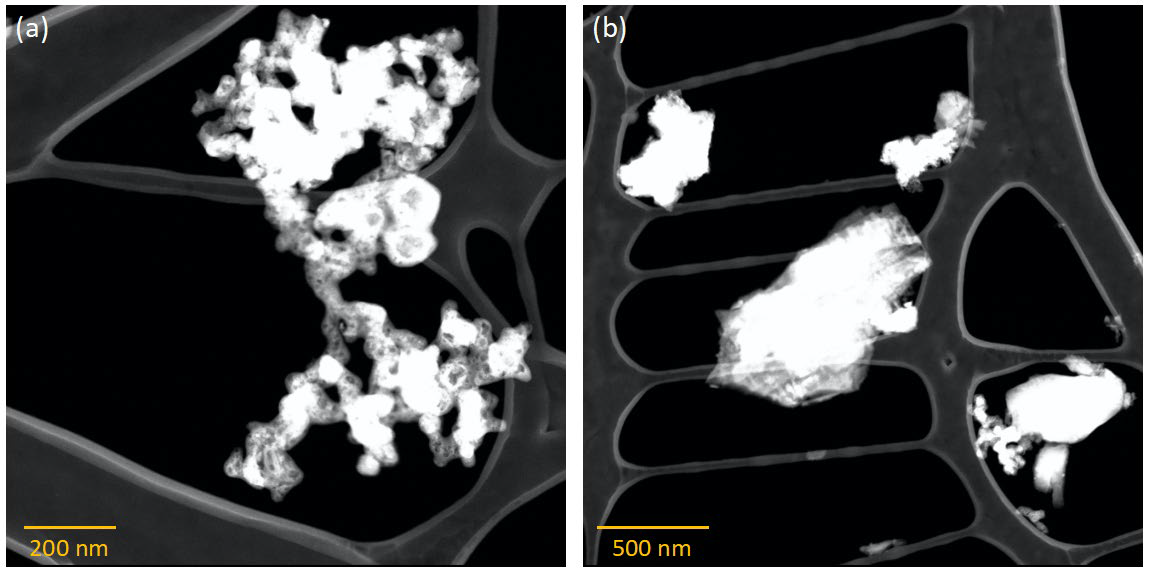
STEM micrographs of synthesized Mg/B solid solution showing B with Mg (darker phase) near the surfaces: (a) smaller and (b) larger particles. Image Credit: Jensen, D et al., ACS Applied Energy Materials
The solid solutions displayed high thermal stability, indicating effective passivation. Additionally, the amount of exothermic heat release from the synthesized solutions remained identical even after six months of storage, indicating an increased shelf life. The synergistic effect of redox reaction between native B oxide and Mg and oxidation of MgB2, B, and Mg enhanced the heat release from B/Mg solid solutions to almost 90% of the theoretical energy density of B/Mg systems.
To summarize, the findings of this study demonstrated the feasibility of using B/Mg solid solutions for energetic applications and the effectiveness of the dry powder-based technique in developing energetic materials with enhanced heat release and reactivity.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.
Source:
Jensen, D., Matsoukas, T., Chen, C-H. et al. Synthesis and Characterization of Magnesium/Boron Solid Solutions for Energetic Applications. ACS Applied Energy Materials 2022. https://doi.org/10.1021/acsaem.2c00312