Plastic upcycling is progressing mainly due to a newly designed catalyst for breaking down plastics. A team of researchers led by experts at Ames Laboratory produced the first processive inorganic catalyst to break down polyolefin polymers into chemicals to make more valuable products in 2020.
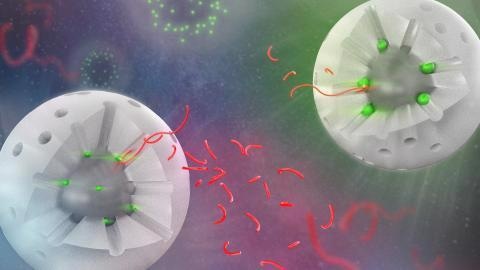 Visual of two variations of the catalyst, with a segment of the shell removed to show the interior. The white sphere represents the silica shell, the holes are the pores. The bright green spheres represent the catalytic sites, the ones on the left are much smaller than the ones on the right. The longer red strings represent the polymer chains, and the shorter strings are products after catalysis. All shorter strings are similar in size, representing the consistent selectivity across catalyst variations. Additionally, there are more smaller chains produced by the smaller catalyst sites because the reaction occurs more quickly. Image Credit: Ames Laboratory
Visual of two variations of the catalyst, with a segment of the shell removed to show the interior. The white sphere represents the silica shell, the holes are the pores. The bright green spheres represent the catalytic sites, the ones on the left are much smaller than the ones on the right. The longer red strings represent the polymer chains, and the shorter strings are products after catalysis. All shorter strings are similar in size, representing the consistent selectivity across catalyst variations. Additionally, there are more smaller chains produced by the smaller catalyst sites because the reaction occurs more quickly. Image Credit: Ames Laboratory
The team has since devised and tested a plan for accelerating the change without compromising attractive products.
Wenyu Huang, an Ames Lab scientist, was the one who came up with the idea for the catalyst. It contains platinum particles that are supported by a solid silica core and encased in a silica shell with uniform holes that can provide access to catalytic sites.
Due to the high cost of platinum and its restricted supply, the total amount of platinum required is minimal. Long polymer chains thread through the pores and touch the catalytic sites during deconstruction tests, and then the chains are broken into smaller-sized bits that are no longer plastic material.
The team created three variants of the catalyst, according to Aaron Sadow, a scientist at Ames Lab and head of the Institute for Cooperative Upcycling of Plastics (iCOUP). The cores and porous shells of each variant were of the same size, but the diameters of the platinum particles varied from 1.7 to 2.9 to 5.0 nm.
The researchers expected that changes in platinum particle size would alter the lengths of product chains, resulting in longer chains for big platinum particles and shorter chains for small platinum particles. However, the researchers determined that the lengths of the product chains for all three catalysts were the same.
In the literature, the selectivity for carbon-carbon bond cleavage reactions usually varies with the size of the platinum nanoparticles. By placing platinum at the bottom of the pores, we saw something quite unique.
Aaron Sadow, Scientist, Ames Laboratory
Rather, the three catalysts had varying rates of breaking the chains into smaller molecules. The bigger platinum particles interacted more slowly with the lengthy polymer chain, but the smaller ones responded faster.
This greater rate might be due to the smaller nanoparticles’ surfaces having a larger percentage of edge and corner platinum sites. These sites are more active in cleaving the polymer chain than the platinum located in the faces of the particles.
The findings are significant, according to Sadow, since they reveal that the activity can be altered independently of selectivity in these processes.
Sadow added, “Now, we are confident that we can make a more active catalyst that would chew up the polymer even faster, while using catalyst structural parameters to dial in specific product chain lengths.”
This sort of bigger molecule reactivity in porous catalysts is not well explored, according to Huang. As a result, the research is critical for understanding both the underlying science and how it applies to upcycling plastics.
We really need to further understand the system because we are still learning new things every day. We are exploring other parameters that we can tune to further increase the production rate and shift the product distribution. So, there are a lot of new things in our list waiting for us to discover.
Wenyu Huang, Scientist, Ames Laboratory
The paper is co-authored by X. Wu, A. Tennakoon, R. Yappert, M. Esveld, M.S. Ferrandon, R.A. Hackler, A.M. LaPointe, A. Heyden, M. Delferro, and B. Peters, and it is published in Journal of the American Chemical Society.
The Institute for Cooperative Upcycling of Plastics (iCOUP), under the leadership of Ames Laboratory, conducted the study. Scientists from Ames Laboratory, Argonne National Laboratory, UC Santa Barbara, University of South Carolina, Cornell University, Northwestern University, and the University of Illinois Urbana-Champaign constitute the iCOUP Energy Frontier Research Center.
Journal Reference:
Wu. X, et al. (2022) Size-Controlled Nanoparticles Embedded in a Mesoporous Architecture Leading to Efficient and Selective Hydrogenolysis of Polyolefins. Journal of the American Chemical Society doi:10.1021/jacs.1c11694.