With Vitralit® UD 8050 MV F, Panacol has developed another adhesive system certified for use in the medical industry. What is special about this adhesive is that in addition to its primary curing - UV crosslinking - this adhesive also offers secondary moisture post-curing.
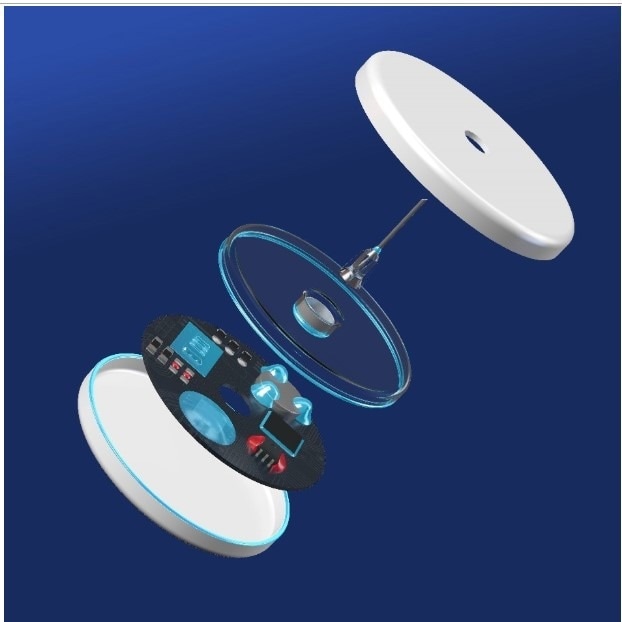
Image Credit: Panacol-Elosol Gmb
This means that components in medical devices can be cured in a process-safe manner and without thermal influence, even if shadow zones are present in component cavities.
Two challenges commonly arise in medical applications when bonding plastics or assembling PCBs and FPCBs. Not every plastic is transparent, which makes it difficult to cure UV adhesives in a process-safe manner.
In most cases, thermal post-curing is relied upon. However, thermal curing often becomes the second challenge. Not all electronic components and plastics can be subjected to thermal stress. In these cases, curing with heat is not a viable option.
To address these challenges, Panacol has developed a new, stress-free adhesive solution specifically for medical device applications. The primary curing of Vitralit® 8050 MV F can be carried out in seconds with UV or LED UV light. LED curing units with a wavelength of 405 nm, such as the LED Spot 100 IC from Hönle, are best suited here.
Secondary curing of the adhesive in the shadow areas that are not reached by the UV/visible light takes place through a moisture curing process. Surface and atmospheric moisture react with the adhesive to initiate a curing process.
Unlike moisture curing cyanoacrylates, Vitralit® UD 8050 MV F can be processed reliably because its primary curing is based on UV crosslinking. Moisture in the air does not cause the adhesive to cure while still in the dispensing needle.
This offers the user a wide process window. In addition, there are no blooming of frosting effects on the plastics, which can occur when using cyanoacrylates.
Vitralit® 8050 MV F is certified according to ISO 10993-5/-10/-23 biocompatibility testing. Since the dual-curing adhesive exhibits high adhesion to many different substrates, it is the optimal solution for a wide variety of bonding applications.
As shown in Fig. 1, the UV acrylate is ideally suited for fixing batteries on glucose sensors (corner bonding). It can also be used as a glob top for protecting SMD components.
The adhesive will cure reliably and stress-free, even in the shadow areas under the bonding wires.
When used for structural bonding, Vitralit® 8050 MV F can be applied between transparent and non-transparent substrates. Bonding is accomplished in seconds with UV/visible light.
Shadowed areas will subsequently post-cure with moisture. Due to the high ionic purity of Vitralit® 8050 MV F, the adhesive can be processed on metallized surfaces without hesitation.
For optical quality control of the bond, Vitralit® 8050 MV F is additionally equipped with fluorescent markers that can be excited with short-wave light.
The latest medical adhesive in Panacol's portfolio thus enables the company to offer a wider range of different adhesive systems for the medical electronics and medical wearables market.
From UV acrylics and UV epoxies to electrically conductive products and conventional thermosetting epoxies, all applications from structural package bonding to needle bonding and PCB bonding solutions can be provided.
Visit us at Bondexpo in Stuttgart, Germany in hall 5, booth 5417!
About Panacol
Panacol-Elosol GmbH, a Hönle Group company, is an internationally active supplier in the growth market for industrial adhesives with a broad product range from UV adhesives to structural adhesives and conductive adhesives.
Together with Dr. Hoenle AG, parent company of the Hönle Group and the world's leading supplier of industrial UV technology, Panacol presents itself as a reliable systems provider from bonding to curing of adhesives.
Vitralit® 8050 MV F is suitable for bonding on printed circuit boards as well as for bonding housings or syringe needles.