A new paper published in the Journal of Polymer Science has investigated the synthesis of new ionic liquid methacrylates and dimethacrylates. The research has been carried out by scientists from Niederrhein University of Applied Sciences in Germany.
Study: Synthesis and photoinitiated polymerization of new ionic liquid methacrylates. Image Credit: Spunt/Shutterstock.com
Ionic Liquids
Ionic liquids have been proposed in recent years for applications such as fuel cells, batteries, dye-sensitized solar cells, and as solvents for multiple important industrial chemical processes such as polymer and organic synthesis.
Ionic liquids are traditionally defined as salts that exist in their liquid state below the boiling point of water. They possess a significantly lower melting point and glass transition temperature than commonly utilized salts.
However, this traditional definition has some problems, due to the existence of salts that have a melting point slightly above the boiling point of water which is similar to ionic liquids in chemical structure. To overcome the issues with this conventional definition, scientists have defined them as liquids with a pure form composed entirely of ionic constituents.
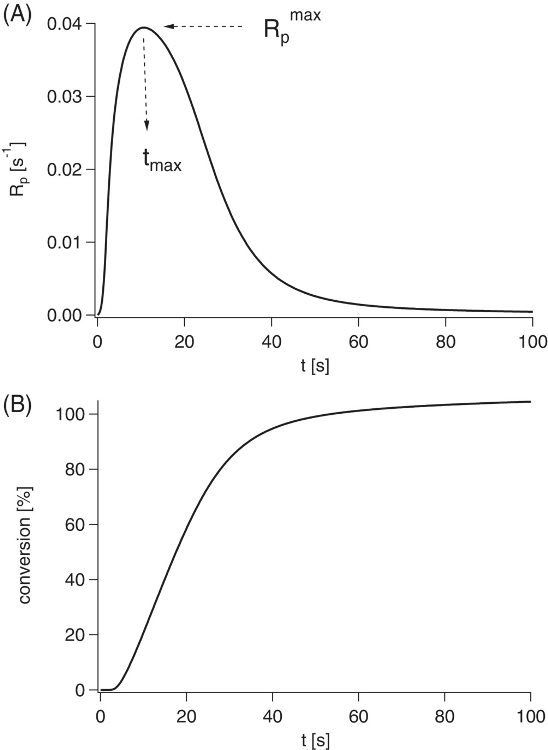
Photoinitiated polymerization of the room temperature ionic liquid methacrylate ILMA-B-NTf2 in bulk at 40 °C using bis-4-(methoxybenzoyl)diethylgermane as photoinitiator and a LED emitting at 395 nm for irradiation. (A) Polymerization rate (Rp) measured by photo-differential scanning calorimetry (DSC) as function of the irradiation time (t); (B) conversion determined from the photo-DSC measurement as function of the irradiation time (t). Image Credit: Strehmel, V et al., Journal of Polymer Science
Ionic Liquid Monomers
Ionic liquids can be modified with polymerizable functional groups, becoming ionic liquid monomers. Ammonium and imidazolium-based ionic liquid monomers with one functional polymerizable component have been widely researched in recent years. Studies have commonly employed bis(trifluoromethylsulfonyl)imide (NTf2) as the anion.
Scientists have evaluated several polymerization techniques to convert ionic liquid monomers into polyelectrolytes. These methods include controlled radical polymerization, enzymatic polymerization, free radical polymerization, and group transfer polymerization. Photoinitiators and conventional thermal initiators have been employed in free radical polymerization methods.
Ionic liquid monomer polymerization methods display varying degrees of environmental friendliness, with photo-initiated and enzymatic polymerization classed as green methods. This is mainly due to the ambient temperature conditions these techniques can operate in.
The Study
The authors have demonstrated the development of new ionic monomers comprising methacrylates and dimethacrylates. Triflate (TFl,) trifluoroacetate (TFAc,) and tris(pentafluroethyl)trifluorophosphate (FAP) were evaluated as alternative anions in these ionic liquids as well as NTF2.
The new ionic liquids presented in the research were compared with ionic monomers which have sufficient information regarding both the monomers and functional polymers that can be derived from them in the current body of literature.
The influence on glass transition temperature and the melting point of the prepared ionic liquid methacrylates was investigated by the authors as well as the polymerization kinetics. Photo-differential scanning calorimetry was employed as an analytical technique to evaluate the samples.
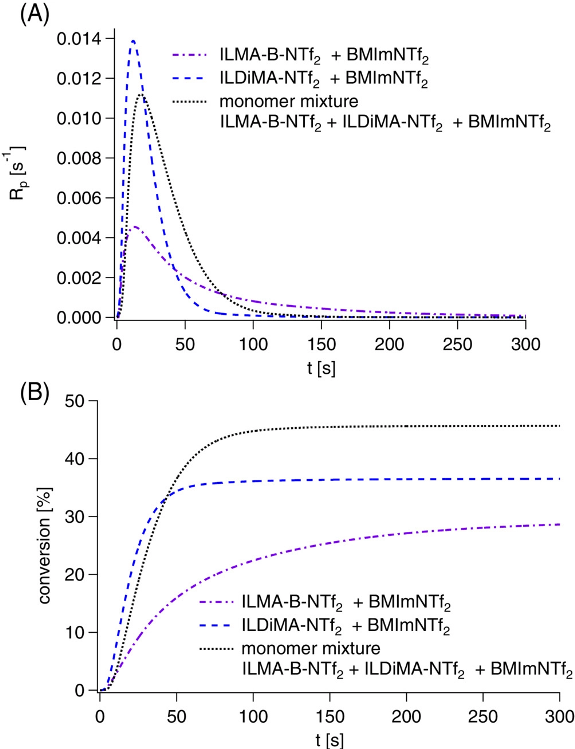
Photoinitiated polymerization of the room temperature ionic liquid monomers ILDiMA-NTf2, ILMA-B-NTf2 and a mixture of the two monomers at 40 °C in the presence of BMImNTf2 using bis-4-(methoxybenzoyl)diethylgermane as photoinitiator and a LED emitting at 395 nm for irradiation. (A) Polymerization rate (Rp) measured by photo-differential scanning calorimetry (DSC) as function of the irradiation time (t); (B) conversion determined from the photo-DSC measurements as function of the irradiation time (t). Image Credit: Strehmel, V et al., Journal of Polymer Science
Study Conclusions
Several key observations were made in the paper. Firstly, the research reveals that anion choice is crucial for controlling the melting point and glass transition temperature. Using NTF2 or FAP as the anion produces room-temperature methacrylates, although only the dimethacrylate containing NTf2 as the anion is considered to be a room-temperature ionic liquid.
TFAc and TFl anions and counter ion exchange result in solid room-temperature methacrylates and dimethacrylates. Selecting FAP as the counter ion results in solid monomers in the dimethacrylate but produces a methacrylate which can be classed as an ionic liquid monomer at room temperature.
The authors have stated in the paper that the lower limit of the ionic liquid monomer’s liquid range is crucial for the selection of the optimal polymerization process, which is either solution or bulk polymerization. Tailoring synthesis routes by selecting certain anions can improve the proper selection of the polymerization method.
NFT2-incorporated methacrylates have good potential as coatings and membranes. This is due to their quantitative bulk conversion after a brief period of irradiation, which can be as little as ten minutes. However, their use for these applications can be hindered by the resulting polymer’s good solubility.
A more rational choice of liquid ionic methacrylate for these applications could be dimethacrylates incorporating NTf2 or methacrylates containing FAP or NTf2. This is due to the production of insoluble crosslinked polymers by fast photoinitiated polymerization methods. A potential use of these ionic liquid monomers aside from membranes and coatings is in 3D printing.
NTf2 and FAP are potentially more suitable anions in photoinitiated polymerization methods compared to TFAc and TFl due to more rapid polymerization and greater final conversion. Depending on anion selection, the photopolymerization kinetics and the final conversion yield vary in different monomers and mixtures of monomers.
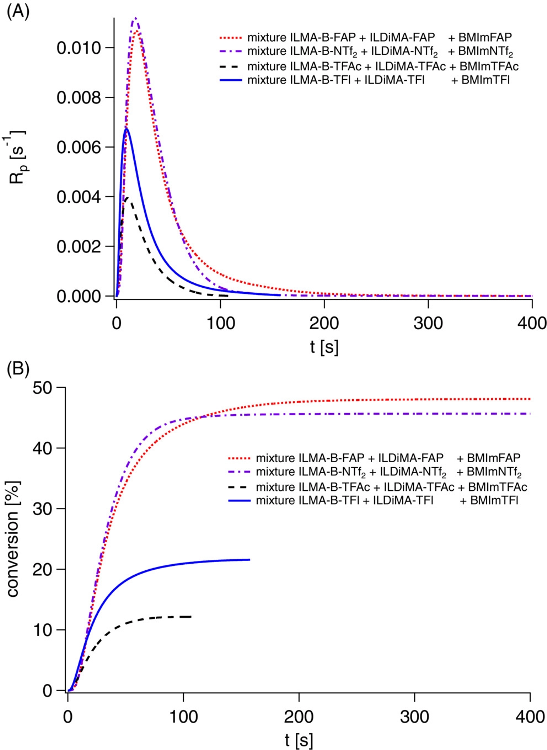
Photoinitiated polymerization of ionic liquid dimethacrylate ionic liquid methacrylate mixtures in ionic liquids comprising the same anion as the ionic liquid monomers: (ILDiMA-NTf2 + ILMA-B-NTf2 + BMImNTf2; ILDiMA-FAP + ILMA-B-FAP + BMImFAP; ILDiMA-TFl + ILMA-B-TFl + BMImTFl; ILDiMA-TFAc + ILMA-B-TFAc + BMImTFAc) at 40 °C using bis-4-(methoxybenzoyl)diethylgermane as photoinitiator, and a LED emitting at 395 nm for irradiation. (A) Polymerization rate (Rp) measured by photo-differential scanning calorimetry (DSC) as function of the irradiation time (t); (B) conversion determined from the photo-DSC measurements as function of the irradiation time. Image Credit: Strehmel, V et al., Journal of Polymer Science
In Conclusion
The paper has revealed the influence of anion choice on the polymerization of ionic liquid monomers and has produced novel ionic liquid methacrylates and dimethacrylates. The choice of anion influences both the melting behavior and polymerization kinetics of these industrially important materials.
The current work provides important information for future research in developing ionic liquid monomers and their use in various processes and manufacturing. The design of a tailored photopolymerization system wherein only the anion is exchanged is made possible by this paper’s experimental findings.
Further Reading
Strehmel, V, Kaestner, P.I & Strehmel, B (2022) Synthesis and photoinitiated polymerization of new ionic liquid methacrylates Journal of Polymer Science [online] onlinelibrary.wiley.com. Available at: https://onlinelibrary.wiley.com/doi/10.1002/pol.20220361
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.