Scientists from Poland’s Research and Innovation Centre Pro-Akademia have reviewed the progress in plasma-driven solution electrolysis solutions for hydrogen production, a key green energy technology. Their research has been published in the journal Energies.
Study: Overview of the Hydrogen Production by Plasma-Driven Solution Electrolysis. Image Credit: Shawn Hempel/Shutterstock.com
Hydrogen: A Sustainable Fuel of the Future
The urgent need to address the climate change and energy crises have focused the attention of researchers on low-carbon, high-energy-density alternative and sustainable fuels which can meet the demands of modern industry and transportation.
Amongst the various alternative fuel and energy solutions proposed, hydrogen has emerged as a suitable candidate due to its extremely high specific energy density of around 121 MJ/kg. Furthermore, hydrogen is regarded as a clean energy carrier compared to traditional fossil fuels such as oil, coal, and gas.
Another key advantage of hydrogen as a green energy and fuel solution is its ability to ensure energy security compared to renewable energy such as solar, wind, and hydropower. Hydrogen production is not affected by issues such as seasonality or source fluctuations. This energy source offers continuous conversion of energy sources into hydrogen for fuel cells, industry, and vehicles.
The current methods of hydrogen production rely overwhelmingly on hydrocarbons, such as natural gas and coal, which is at odds with the reputation of hydrogen as a clean energy and fuel source. Around 50% of total global hydrogen production is produced from natural gas via processes such as steam reforming.
Aside from using a non-renewable source, challenges associated with this production route include the high energy and cost demands and carbon dioxide emissions. In order to improve the environmentally friendly and sustainable credentials of hydrogen production, research has intensified into the synthesis of “green” hydrogen from renewable resources such as solar and wind.
Some of these green production routes, such as thermochemical methods, photobiological methods, and electrolysis, are fairly mature, but there is an urgent need to improve their efficiency, increasing their yield and, consequently, lowering their associated costs.
![Influence of the weight concentration of KOH and NaOH on the specific conductivity of their aqueous solutions at 15 °C and 18 °C, respectively. The diagram was built based on the tabular data presented in [27].](https://www.azom.com/images/news/ImageForNews_60229_16656539472497899.png)
Influence of the weight concentration of KOH and NaOH on the specific conductivity of their aqueous solutions at 15 °C and 18 °C, respectively. Image Credit: Bespalko, S & Mizeraczyk, J, Energies
Plasma-driven Solution Electrolysis
Suitable hydrogen production technologies are determined by their cost-effectiveness, hydrogen yield, and, increasingly, their sustainability and environmental friendliness.
In electrolysis methods, various parameters are applied to evaluate their performance, such as their Faradic efficiency and hydrogen yield parameters. Amongst the various proposed green technologies for hydrogen synthesis, plasma-driven solution electrolysis has emerged as a suitable strategy. Studies have demonstrated that this type of electrolysis possesses much higher Faradic efficiency than Faradic electrolysis.
A nontypical electrochemical process, plasma-driven solution electrolysis involves the formation of electric plasma by glow discharges from direct or pulsed currents in gas-vapor envelopes. These envelopes are produced in the vicinity of discharge electrodes submerged in electrolytic solutions. This method produces significantly enhanced yields of chemical products and can synthesize more chemical compounds than Faradic electrolysis.
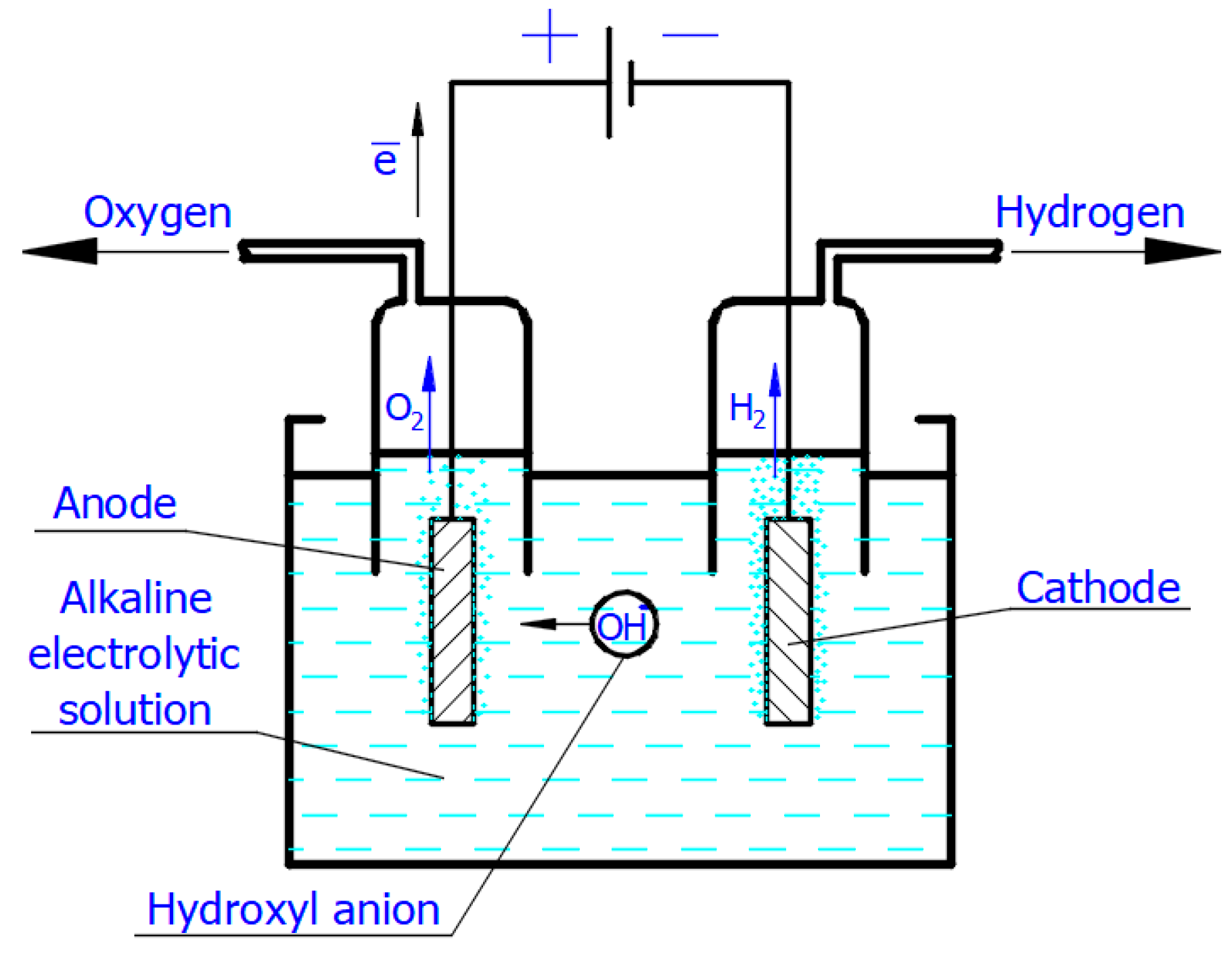
Scheme of the simplest alkaline electrolytic cell illustrating production of H2 and O2 according to Equations (2) and (4). The scheme shows only hydroxyl anions participating in the charge transport. Image Credit: Bespalko, S & Mizeraczyk, J, Energies
The Review Paper
A timely and comprehensive review of this plasma-driven solution electrolysis for hydrogen production is needed due to the method’s potential and the urgent need for high-efficiency, high-yield, and industrial-scale hydrogen manufacturing processes. Ninety-one papers have been reviewed. Several findings have been revealed in the paper.
Firstly, several physiochemical and physical processes must occur in sequence before plasma-driven electrolysis in an electrolyte solution: Faradic electrolysis, Joule heating, solvent evaporation, gas-vapor envelope formation, ionization of the mixture, and electrical discharge induction. Plasma-driven solution electrolysis regimes can be anodic or cathodic depending on the smaller electrode’s charge.
Complex physiochemical and physical processes occur in both anodic and cathodic regimes, leading to decomposition into hydroxyl groups and hydrogen: Faradic electrolysis, ion-impact decomposition, thermal decomposition, photodecomposition, and electron-impact decomposition. Reverse hydroxyl radical reactions can hinder hydrogen production due to the formation of hydrogen peroxide.

Illustration of the regions at the discharge electrode. Image Credit: Bespalko, S & Mizeraczyk, J, Energies
Several parameters have been highlighted in the paper which influence hydrogen production using plasma-driven solution electrolysis. These include the electrolytic solution’s temperature, electrolytic solution concentration, the discharge electrode’s immersion depth, organic additive presence, and applied voltage.
Plasma-driven solution electrolysis possesses several notable advantages over other forms of electrolysis for hydrogen production. This method produces a 3.9 times higher energy yield than methods such as alkaline electrolysis, solid oxide electrolysis, and polymer electrolyte membrane hydrolysis.
Other advantages include the high diffusion of produced hydrogen, low losses of resistance, and no need for precious metal catalysts. However, there are some disadvantages, such as the need for gas separation methods to provide high-efficiency removal of hydrogen from gas mixtures and limited reported energy efficiency.
Finally, the paper makes recommendations for the future of hydrogen production using plasma-driven solution electrolysis. For instance, energy efficiency needs to be increased, and stable materials are needed to increase the discharge electrode’s lifetime. Additionally, research into catalysis could improve the efficiency of hydrogen production. Whilst there are challenges, this electrolysis method is highly promising.
Further Reading
Bespalko, S & Mizeraczyk, J (2022) Overview of the Hydrogen Production by Plasma-Driven Solution Electrolysis Energies 15(20) 7508 [online] mdpi.com. Available at: https://www.mdpi.com/1996-1073/15/20/7508
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.