In a paper recently published in the journal Batteries & Supercaps, researchers presented an electrospun membrane of polymer electrolyte synthesized using a polymer electrolyte with a composition in a 36:1 ratio of poly(ethyleneoxide) and magnesium bis(trifluoromethanesulfonyl) imide (PEO:Mg(TFSI)2 36:1).

Study: Fast Magnesium Conducting Electrospun Solid Polymer Electrolyte. Image Credit: sfam_photo/Shutterstock.com
Background
Since the invention of Lithium-Ion-Batteries (LiB), there has been an increasing demand to store vast quantities of electrical energy. The divalent Mg2+ ion, with its widespread availability and high specific volumetric and gravimetric capacities, offers great promise. Furthermore, the lower reducing nature of Mg metal and its less conspicuous tendency to develop dendrites make Mg metal anodes better suited for use with a wide range of polymers and solvents.
A complete cell with MgMn2O4 cathode and Mg metal anode is operable in an electrolytic solution of 0.5M Mg(ClO4)2 in acetonitrile. Aside from liquid electrolytes, numerous gel polymer electrolytes (GPEs) have shown satisfactory conductivities. Inorganic fillers, in addition to ionic liquids (ILs), can be used to improve the electrochemical characteristics of solid polymer electrolytes (SPEs). The present study provided results from the electrospinning synthesis of a new solid-state Mg(TFSI)2:PEO electrolyte.
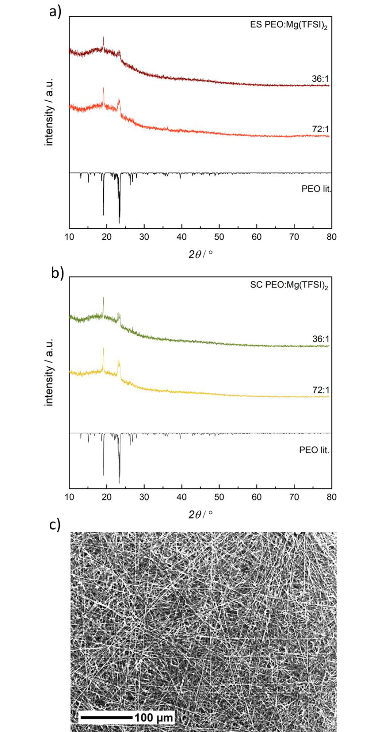
P-XRD of a) electrospun (ES) and b) solution casted (SC) PEO:Mg(TFSI)2 SPEs with different molar compositions compared to a crystalline phase of PEO from literature, 34 Literature data are drawn with negative intensities. c) SEM image of an electrospun PEO:Mg(TFSI)2 36:1 SPE with 250-fold magnification. Image Credit: Walke, P et al., Batteries & Supercaps
About the Study
In this study, the team studied two distinct compositions, 72:1 and 36:1, both created by solution casting and electrospinning. The addition of succinonitrile (SN) within the membranes was also attempted. However, a minor amount of SN was absorbed inside the membranes.
Custom electrospinning equipment was used to manufacture the fibrous membranes. The produced solution was then transferred via a capillary to achieve all the solid PEO:Mg(TFSI)2 polymer electrolyte samples. A grounded ring collector was used to collect the membrane fibers.
A diffractometer was used to perform powder XRD analyses on the chosen membranes. Differential Scanning Calorimetry (DSC) was used in Al-crucibles to test the thermal characteristics of the chosen membranes. Impedance spectroscopy at a 20-mV amplitude was used to assess the ionic conductivity of the membranes in a frequency ranging from 10 Hz to 10 MHz. Furthermore, using 2032-type coin cells, the electrochemical cycling was carried out in symmetric cells. A BRUKER Avance III spectrometer in conjunction with a 7 T magnet was utilized for investigating the nuclear magnetic resonance in the solid state. Additionally, a scanning electron microscope was used to examine the structure of the produced membranes' fibers.
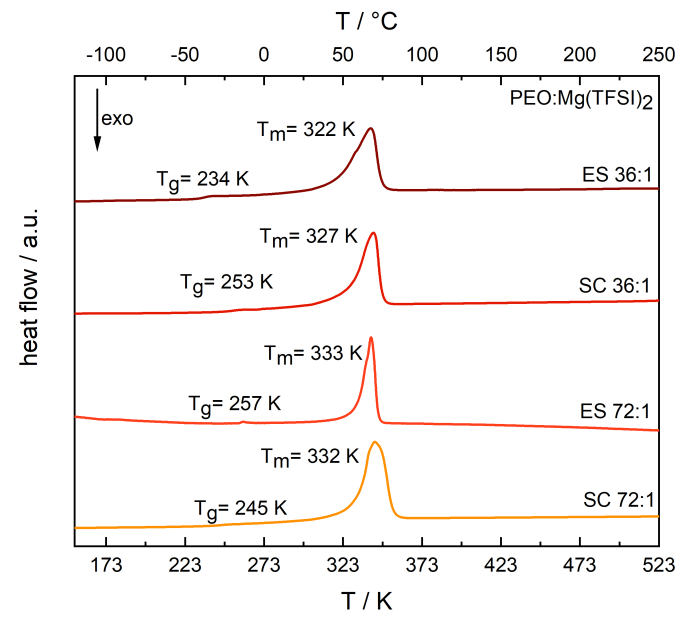
DSC curve of electrospun PEO:Mg(TFSI)2 SPEs at different molar compositions and their fitted melting points Tm and glass transition temperatures Tg. Image Credit: Walke, P et al., Batteries & Supercaps
Observations
The submicrometric fibers’ amorphous membranes were generated by electrospinning PEO:Mg(TFSI)2 solutions with varied molar compositions. No reflections suggesting the presence of organized Mg(TFSI)2 were observed in the P-XRD tests. The melting temperature of the electrospun PEO:Mg(TFSI)2 72:1 decreased to 322 K from 333K.
The conductivity of the electrospun membrane with a molar proportion of PEO:Mg(TFSI)2 of 72:1 was 6.0x10-8 Scm-1 and 1.0x10-5 Scm-1 at 273 K and 323 K, respectively. At 273 K, maximum ionic conductivity was attained with a PEO:Mg(TFSI)2 36:1 SPE measuring 1.8x10-6 Scm-1. This validated the electrospun SPEs' improved performance over SPEs produced with solution casting. The electrospun sample consistently had the least glass transition temperature and was directly attributable to PEO chain mobilities.
The energy-dispersive X-ray spectroscopy (EDX) scans showed a uniform distribution of F and Mg throughout the membrane. Cyclic voltammetric analysis on the 36:1 sample between the two Mg metal electrodes demonstrated the potential of PEO:Mg(TFSI)2 SPEs in transporting the Mg ions across the membrane. The carbon 13 nuclear magnetic resonance (13C-NMR) was used to study the motion of the PEO chains inside the membrane. At room temperature, the 13C-NMR data revealed the initiation of PEO chain mobility. Moreover, with the rising temperature, the total signal strength increased, while the lines blurred into a single signal, which was lost at room temperature. However, when compared to the electrospun monovalent systems such as PEO:LiTFSI and PEO:LiBF4, the initiation of the motional process occurred at rather higher temperatures. This could imply that the divalent Mg2+ cation strongly coordinated with the ether oxygen atoms.
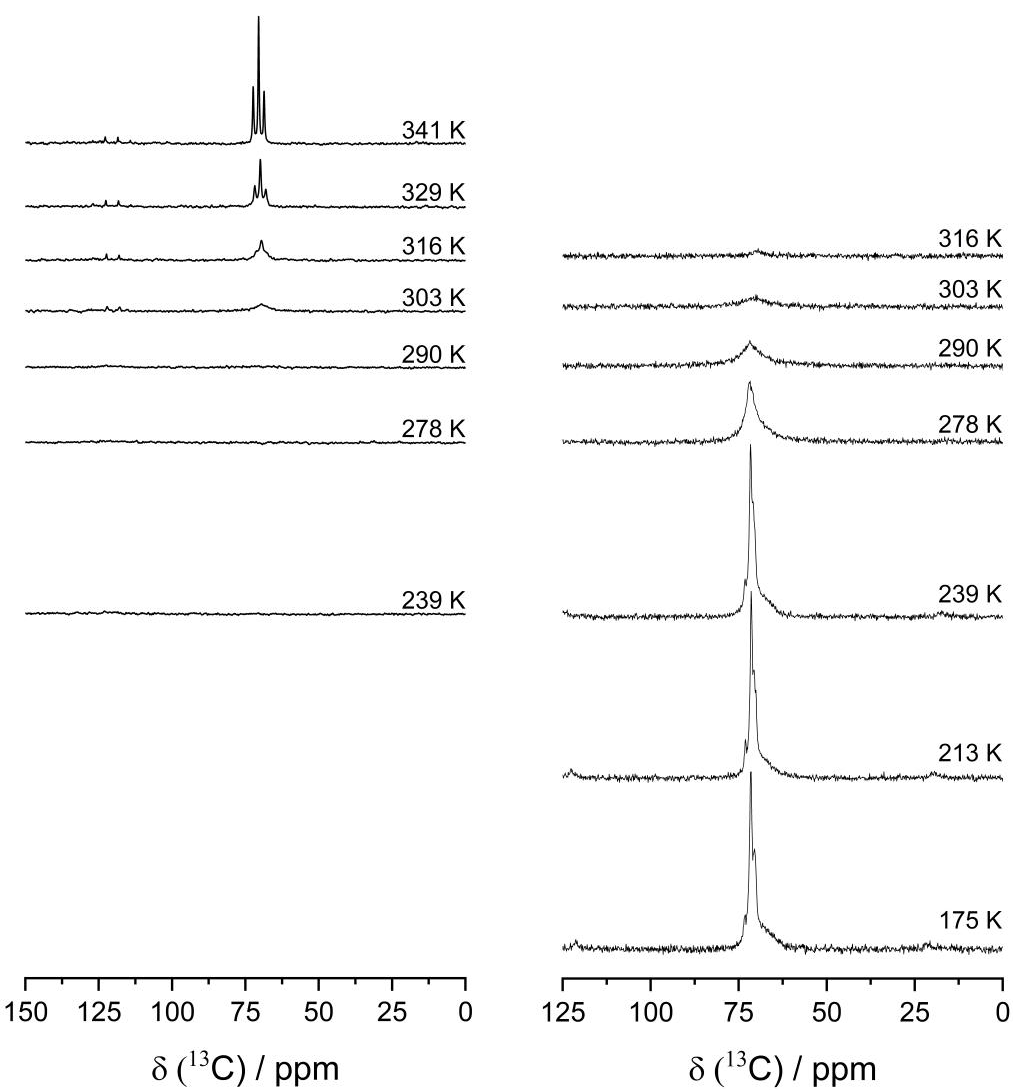
Temperature-dependent 13C-MAS spectra (left) and 13C-{1H}-CP-MAS spectra (right) for the PEO:Mg(TFSI)2 36:1 membrane for the indicated temperatures. Image Credit: Walke, P et al., Batteries & Supercaps
Conclusions
To summarize, the researchers established that without using any organic, inorganic, or liquid additive, the electrospun-synthesized solid PEO-based polymer electrolyte PEO:Mg(TFSI)2 36:1 exhibited a 2.0x10-5 Scm-1 ionic conductivity at room temperature. The electrospun samples' ionic conductivity was also greater than the samples produced by solution casting. Furthermore, solution casting and electrospinning can be used to prepare SPEs of PEO:Mg(TFSI)2 with various molar compositions. In symmetrical Mg cells, the conducted impedance spectroscopy and cyclic voltammetry tests revealed that the Mg2+ ions play a role in enhancing ionic conductivity.
Because of the well-known problem of the formation of the electrode-SPE-interface resistance owing to passivation processes, the cyclic voltammetry current was low. According to the authors, this work highlights the promise of Mg ion electrolytes along with the contribution of electrospinning to the observed conductivity enhancement when transitioning to electrospinning from solution casting.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.
Source:
Walke, P., Venturini, J., Spranger, R..J., van Wüllen, L. and Nilges, T. (2022), Fast Magnesium Conducting Electrospun Solid Polymer Electrolyte, Batteries & Supercaps, 2022, e202200285, DOI: https://doi.org/10.1002/batt.202200285