 By Surbhi JainReviewed by Susha Cheriyedath, M.Sc.Oct 26 2022
By Surbhi JainReviewed by Susha Cheriyedath, M.Sc.Oct 26 2022In an article recently published in the open-access Polymer Journal, researchers discussed semiconducting polymer synthesis by synergistic catalysis.

Study: Synergistic catalysis for the synthesis of semiconducting polymers. Image Credit: Photobank.kiev.ua/Shutterstock.com
Background
The potential use of organic semiconducting polymers in organic light-emitting diodes, sensors, organic photovoltaics, photodetectors, organic field-effect transistors, and more recently bioelectronics, has been the subject of extensive research over the past few decades. The creation of increasingly complicated organic semiconducting polymers has made it possible to research the aforementioned organic electronic devices, but it has also increased environmental burdens and raised polymer synthesis costs.
Cross-coupling reactions with metal catalysts are commonly used to create organic semiconducting polymers. Although these reactions allow for strong transformations, pre-functionalization of the monomers is necessary, adding extra steps to the total synthesis.
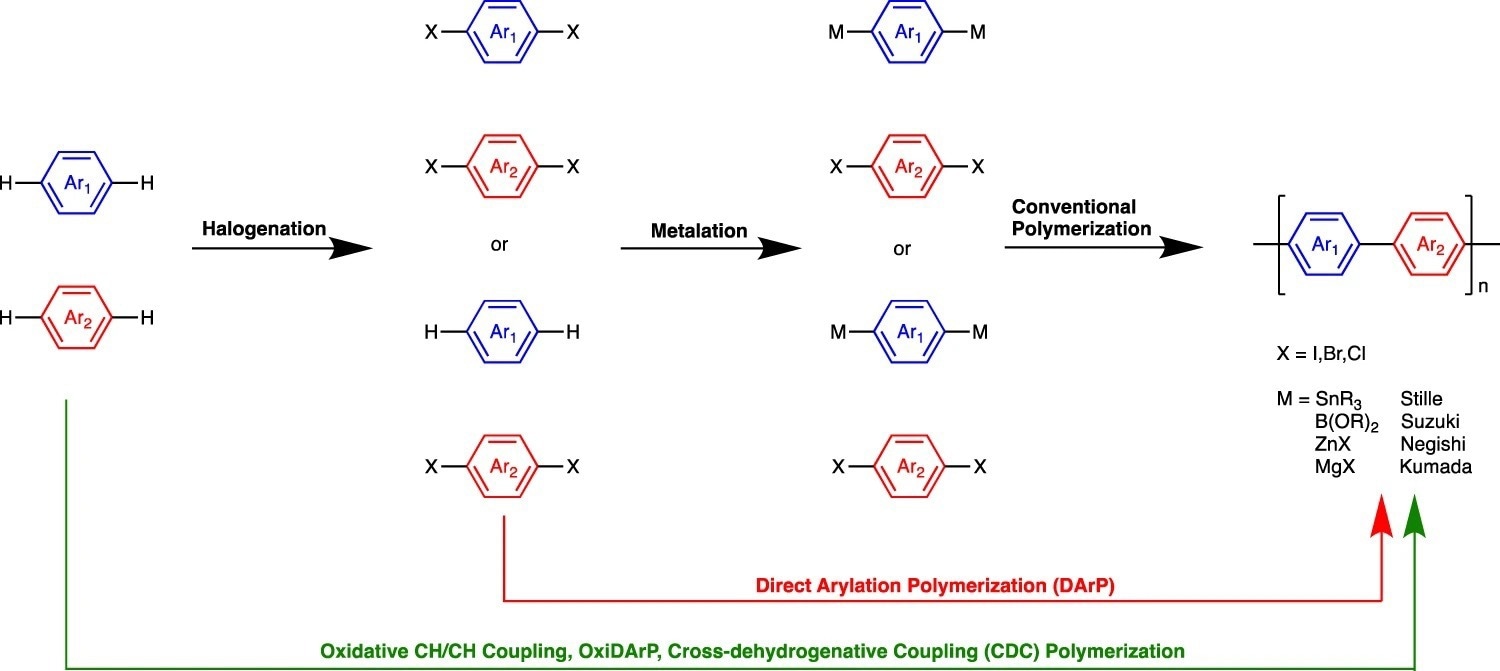
Routes to the synthesis of alternating semiconducting polymers. The conventional route is shown with black arrows, where the halogenation and metalation of precursors are required to generate monomers for polymerization. The red arrow depicts direct arylation polymerization (DArP) where halogenation of one of the monomers is needed. Finally, the green arrow depicts oxidative CH/CH coupling or cross-dehydrogenative coupling (CDC) polymerization where no prefunctionalization is needed. Image Credit: Luscombe, C. K et al., Polymer Journal
For solving the problems associated with more traditional metal-catalyzed cross-coupling reactions, direct arylation polymerizations (DArP) and oxidative CH/CH coupling has received much attention. The regioselectivity problems in these reactions can result in cross-linking and branching, and homocoupling inserts flaws into the polymer backbone. By activating two separate substrates with at least two different catalysts, synergistic catalysis enables previously impossible transformations. Although synergistic catalysis can be advantageous, there aren't many examples of it since it can be challenging for catalysts to preferentially activate one substrate over the other and because monocatalytic side reactions should be avoided.
About the Study
In this study, the authors discussed recent efforts to create more effective synthetic pathways for the development of organic semiconductors with high-performance characteristics such as high charge mobility and high power conversion efficiencies for applications in organic photovoltaics.
The team specifically discussed the idea of synergistic catalysis, which entailed the employment of two or more catalysts with orthogonal reactivity to permit reactions that were not achievable with the use of a single catalyst. Synergistic catalysis opened the door to more time-, cost-, labor-, and energy-efficient ways for the manufacturing of semiconducting polymers by enabling controlled polymerizations, room-temperature reactions, and polymerizations with improved regioselectivity.
The researchers investigated the employment of two catalysts to achieve reactivities that were not previously possible to address the difficulties associated with gaining selectivity and control over polymerization operations. The utilization of synergistic catalysis, especially dual transition metal synergistic catalysis, for the synthesis of semiconducting polymers was the main goal of this study, with considerable emphasis on recent developments made by the team in this field.
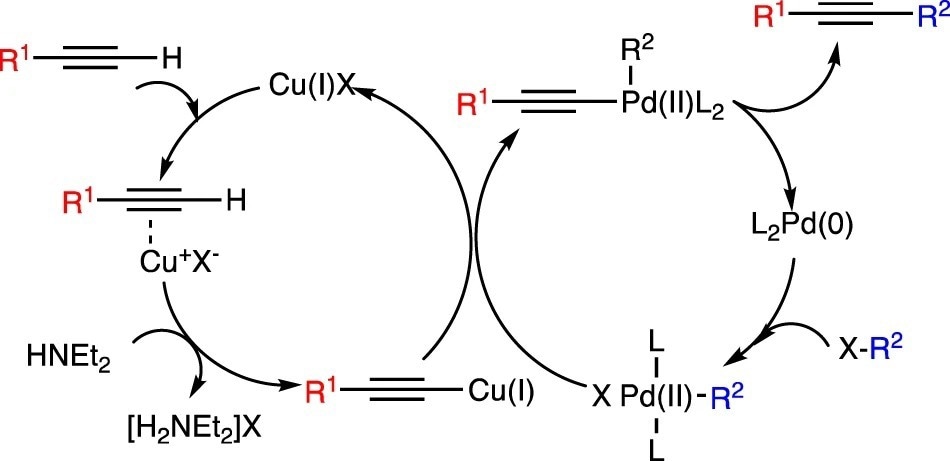
Sonogashira cross-coupling reactions of alkynes and aryl iodides. Image Credit: Luscombe, C. K et al., Polymer Journal
Observations
The coordination of Cu caused simultaneous activation of the alkyne (I). Cu(I) was regenerated as a result of transmetalation between the two active substrates. Reductive elimination, which also produced the cross-coupled product, was used to regenerate Pd(0). The monometallic variants of the reaction either required the usage of a stoichiometric amount of metal or high temperatures. Dibrominated and bisalkynyl monomers were combined in a Sonogashira reaction to produce polymers with a moderate degree of polymerization (DP) and cross-linking.
The proposed mechanism worked by transmetalation of the organostannane with the copper catalyst for the Pd/Cu catalytic form. The Pd catalytic cycle substituted the transmetalation of the organostannane with the transmetalation of Cu (I). Polythiophenes, polyphenylenes, and polyfluorenes could all be synthesized under controlled conditions using catalyst transfer polymerizations (CTPs). Control of the polymerization was accomplished through the development of a π-aryl complex, regardless of the stoichiometric organometallic reagent.
The optimal Pd source was identified through catalyst screening, and it was discovered that Pd-PEPPSI-iPr enabled a polymerization with live features, in which chain extension occurred with the addition of an additional monomer after the first monomer was consumed. Additionally, a linear correlation between the polymerization and the number-average molecular weight (Mn) and monomer conversion was observed. The dispersity (Đ) was between 1.0 and 1.3.
The benzofuran reaction was discovered to not be light-sensitive or radical-mediated and instead appeared to be a Heck reaction. It was observed that the Pd-mediated CH activation of thiophene was the rate-determining step and that the initial cross-coupling sequence to form a cross-coupled dimer was slow. The rate-determining step in the second cross-coupling sequence was the reoxidation of Pd(0) to Pd(II). There was a significant degree of homocoupling present, and the percentage alternation was only 67–76%, whereas 100% alternation indicated no homocoupling. To remove the proton during the synthesis of the Au-thiophene monomer, a stoichiometric quantity of base was needed.
The creation of an Au(I)-OR bond was required to drive transmetalation since a stoichiometric amount of base resulted in the coordination of an alkoxide or hydroxide to Pd. However, the strength of the Au(I)-OR bond was insufficient to accomplish so.
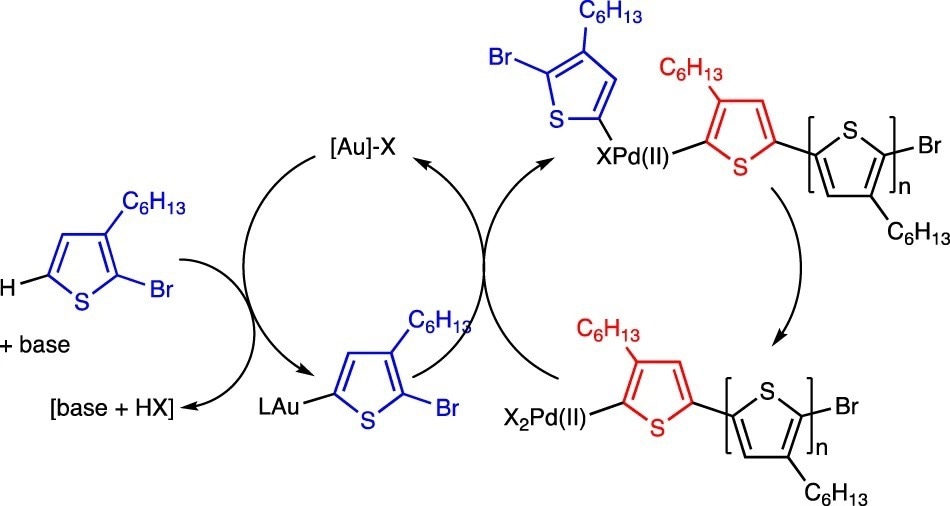
Ideal mechanism for an Au/Pd-mediated CTP. Image Credit: Luscombe, C. K et al., Polymer Journal
Conclusions
In conclusion, this study elucidated the synergistic catalysis in polymerizations. Its significance in providing a way to better manage various polymerization parameters including molecular weight and regioselectivity was demonstrated.
The authors mentioned that a thorough analysis of the unexpected outcomes of polymerizations provided insight into small-molecule reactions, enabling one to identify a novel mechanism at work in the process. They stated that closer partnerships between synthetic organic chemists and polymer chemists would be crucial to promote the development of new polymerization techniques as synergistic catalysis in small-molecule reactions increases.
References
Luscombe, C. K., Phan, S., Sanskriti, I., Synergistic catalysis for the synthesis of semiconducting polymers. Polymer Journal (2022). https://www.nature.com/articles/s41428-022-00719-8
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.