Reviewed by Alex SmithNov 7 2022
In 1842, the renowned British researcher Michael Faraday came up with an incredible observation by chance: A thin layer of water developed on the surface of ice. However, this occurred when the temperature was considerably less than zero degrees; thus, beneath the melting point of ice. Yet, the surface of the ice still melted. This liquid layer on ice crystals is why snowballs tend to stay together.
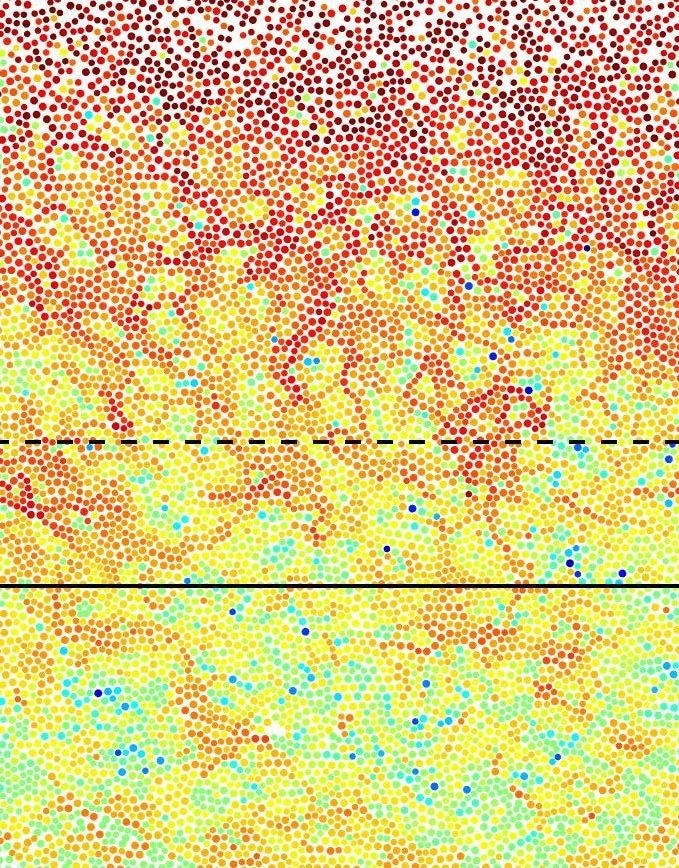 Microscope image of surface melting of glass in colloidal system. Highly mobile particles (red) mark the meting process at the surface. Remarkable is the fact that these highly dynamic regions extend far into the material and thus influence its properties. Image Credit: Research Team Bechinger, University of Konstanz.
Microscope image of surface melting of glass in colloidal system. Highly mobile particles (red) mark the meting process at the surface. Remarkable is the fact that these highly dynamic regions extend far into the material and thus influence its properties. Image Credit: Research Team Bechinger, University of Konstanz.
It was not until nearly 140 years later, in 1985, that this so-called “surface melting” could be scientifically verified under-regulated laboratory conditions.
So far, surface melting has been illustrated in a range of crystalline materials and is scientifically understood clearly: Various degrees below the actual melting point, a liquid layer only a few nanometres thick develops on the surface of the various solid materials.
As the surface properties of materials tend to play a vital role in their use, for example, sensors, battery electrodes, catalysts, and more, surface melting is not only of basic significance but also regarding technical applications.
It should be stressed that this process has completely nothing to do with the effect of, say, taking an ice cube out of the freezer and revealing it to ambient temperature. An ice cube melts on its surface first under such conditions because the surface is considerably warmer compared to the interior of the ice cube.
Surface Melting Detected in Glass
As far as crystals are concerned, with periodically arranged atoms, the thin liquid layer on the surface is normally detected by scattering experiments. This is highly sensitive to the existence of atomic order. As liquids are not arranged in a uniform pattern, these methods can thus clearly solve the look of a thin liquid film on top of the solid.
But this method does not seem applicable for glasses (that is, disordered, amorphous materials) since there is no variation in the atomic order between the solid and the liquid. Hence, the surface melting of glasses has stayed rather undiscovered with experiments.
For these difficulties to be defeated, Clemens Bechinger, a physics professor at the University of Konstanz, and his collaborator Li Tian utilized a trick: rather than studying an atomic glass; they produced a disordered material that has been made up of microscopic glass spheres called colloids. Contrary to atoms, these particles are nearly 10,000 times bigger and could be noted under a microscope in a direct manner.
The scientists could illustrate the process of surface melting in such a colloidal glass since the particles present near the surface move much faster than the solid below. At first glance, such behavior is not completely unforeseen, as the particle density at the surface is lower than the basic bulk material. Hence, the particles close to the surface have more space to move past each other, making them quicker.
A Surprising Discovery
But what surprised Clemens Bechinger and Li Tian, was the fact that even much lower the surface, where the particle density has attained the bulk value, the particle mobility is still considerably higher than the bulk material.
The microscope images display that this earlier unidentified layer is up to 30 particle diameters thick and continues from the surface into the deepest regions of the solid in a streak-like pattern.
This layer which reaches far into the material has interesting material properties since it combines liquid and solid features.
Clemens Bechinger, Professor, Physics, University of Konstanz
As a result, the properties of thin, disordered films rely very much on their thickness.
This property was earlier exploited in their application as thin ionic conductors in batteries, which are discovered to have a considerably greater ionic conductivity than thick films. But with the new knowledge gained from the experiments, this behavior could be realized measurably and improved for technical applications.
Journal Reference:
Tian, L & Bechinger, C (2022) Surface melting of a colloidal glass. Nature Communications. doi.org/10.1038/s41467-022-34317-2.