The electrochemical reduction of carbon dioxide (CO2) to carbon monoxide (CO) has great potential for eliminating CO2 from the atmosphere to lessen pollution and providing alternative energy with carbon monoxide as a component.
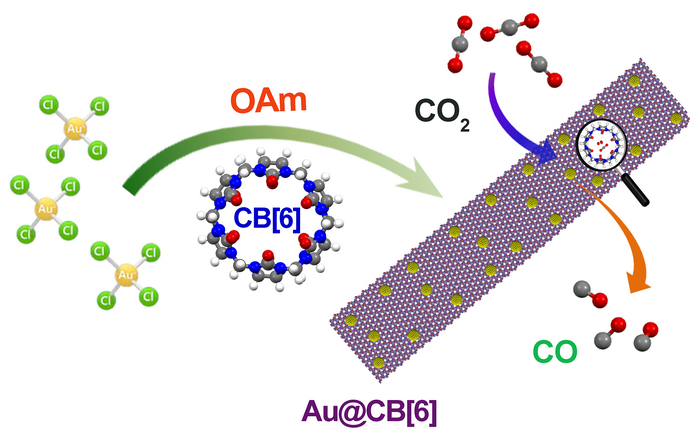 A gold-based hybrid material (Au@CB[6]) is modified by CB[6] in order to efficiently convert CO2 to CO. Image Credit: Nano Research, Tsinghua University Press
A gold-based hybrid material (Au@CB[6]) is modified by CB[6] in order to efficiently convert CO2 to CO. Image Credit: Nano Research, Tsinghua University Press
However, the present electrochemical carbon dioxide reduction reaction (CO2RR) catalysts are neither efficient nor selective enough to make CO2RR a viable alternative.
A group of researchers from the Chinese Academy of Sciences’ Fujian Institute of Research on the Structure of Matter has advanced a gold-based hybrid material by customizing gold nanoparticles with a macrocyclic compound called cucurbit[6]uril (CB[6]), which allows for more efficient CO2RR than was previously possible.
The study was published on December 05th, 2022 in Nano Research journal.
With this work, we hoped to solve the problem of environmental pollution and energy shortage through electrochemical conversion of carbon dioxide to value-added products. In order to enhance the local CO2 concentration on the catalysts’ surface, we utilize macromolecule cucurbit[n]uril to functionalize gold surface, which is the distinguishing feature of our work from those that has been done before.
Minna Cao, Study Corresponding Author, State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences
Minna Cao is also associated with the University of Chinese Academy of Sciences.
Compared to other catalysts, gold is known to be very active in converting CO2 to CO, according to the investigators. However, the binding energy of CO2 and CO to the gold catalyst surface is positively linked, which contradicts the necessity for CO2 adsorption and CO desorption in CO2RR, as CO desorption does not occur due to the positive correlation of its binding energy to the catalyst.
By altering CB[6], the researchers were able to produce a controlled production of nanoparticles. Because CB[6] possesses negatively charged portals and a positively charged surface, the electrical interaction between CB[6] and metal serves to modulate catalytic performance.
The researchers verified both the morphology and surface structure of the nanoparticles through transmission electron microscopy. The gold-based hybrid material (Au@CB[6]) was proven to enhance CO2RR catalytic activity.
We have proved the interaction between cucurbit[6]uril and CO2 through operando electrochemical measurement and density functional theory calculations.
Minna Cao, Study Corresponding Author, State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences
Several factors, according to the investigators, lead to better catalytic performance. First, by accumulating CO2, the CB[6] can enhance the local CO2 concentration near the metal surface, implying that the Au@CB[6] possesses tunable, or adjustable, CO2 enrichment.
Furthermore, by breaking the previously indicated scaling relations of the binding affinity between the catalyst surface and CO2/CO, the alteration of CB[6] allows for enhanced CO2RR.
Furthermore, one reason that CO2RR was previously limited in efficiency with gold surface catalysts was CO2’s low solubility in aqueous electrolytes, which the investigators solved by using the extremely specific binding force of macrocycle to selectively adsorb specific species to regular the electrocatalytic reaction.
The results showed that CB[6] can gather CO2 and lead the increased local CO2 concentration near the metal interface, as well as promote CO desorption, which are the dominating reasons for enhanced CO2RR performance. Using the rigid macrocycle cucurbit[n]uril to modify the catalysts’ surface is a promising pathway to enhance the electrocatalytic performance.
Minna Cao, Study Corresponding Author, State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences
The researchers intend to continue modifying the catalyst to improve the CO2RR’s efficacy.
“In the next step, we hope to adjust the shape and size of the gold catalyst in the presence of cucurbit[n]uril to further promote the catalytic performance toward electrochemical reduction of carbon dioxide to value-added products,” Cao concludes.
The other authors of the paper are Huimin Wang, Yuqing Fu, Zhe-Ning Chen and Wei Zhuang, from the State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, and of the University of Chinese Academy of Sciences.
Rong Cao, the project leader, is affiliated with both the earlier-mentioned institutes as well as the Fujian Science & Technology Innovation Laboratory for Optoelectronic Information of China.
The National Key R&D Program of China, the NSFC, and the Fujian Science & Technology Innovation Laboratory for Optoelectronic Information of China funded this research.
Journal Reference:
Wang, H., et al. (2022) Tunable CO2 enrichment on functionalized Au surface for enhanced CO2 electroreduction. Nano Research. doi.org/10.1007/s12274-022-5159-8.