The valuable metal platinum is the main catalyst for the chemical reactions occurring at the heart of the next generation of highly small and high-performing hydrogen fuel cells.
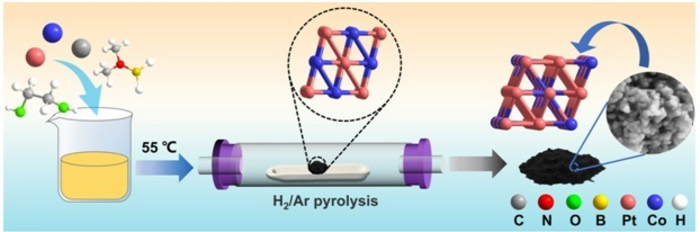 Highly ordered Pt3Co nanoparticles were prepared by a two-step reduction strategy. The half-wave potential of Pt3Co/C reached 0.87 V, showing excellent ORR performance. Image Credit: Zhonghua Xiang, Beijing University of Chemical Technology
Highly ordered Pt3Co nanoparticles were prepared by a two-step reduction strategy. The half-wave potential of Pt3Co/C reached 0.87 V, showing excellent ORR performance. Image Credit: Zhonghua Xiang, Beijing University of Chemical Technology
However, the high cost of platinum is avoiding the extensive adoption of this technology. However, scientists have engineered a nano-scaled platinum and cobalt alloy to use it as a catalyst. This helps in acutely decreasing the amount of platinum required to obtain the same—or even better—performance.
An explanation of his novel platinum-cobalt electrocatalyst and the method utilized to produce it was reported in the journal Particuology on December 15th, 2022.
Hydrogen fuel cells will be required in the clean transition for those parts of the economy, especially heavy transport, which are difficult to electrify with the help of battery technology.
Unluckily, the most generally utilized fuel cell, the so-called alkaline fuel cell, remains to be pretty heavy. This helps restrict its application in those sectors such as shipping and aviation, where space is considered a premium.
The supposed next generation of the fuel cell, the proton exchange membrane fuel cells (PEMFCs—at times known to be the polymer electrolyte membrane fuel cell), is much more compact.
Regrettably, the primary catalyst—substances that help expedite chemical reactions—that have been utilized in an important reaction involved in PEMFCS (the oxygen reduction reaction, or ORR) is the unique and thus costly metal platinum.
Earlier, the high cost of platinum is known to be one of the greatest barriers to extensive PEMFC adoption. As per the data gathered from the US Department of Energy, platinum-group metal catalysts in fuel cells are currently considered to be over 40% of their cost. Certainly, half of all platinum production in the world is utilized by the automobile sector.
This means that even as the high cost of platinum is limiting adoption of fuel cells in vehicles, should any wider adoption occur, this would only exacerbate the problem as there would be even greater demand, and thus higher prices, for this rare metal.
Zhonghua Xiang, Study Author and Electrochemist, Beijing University of Chemical Technology
Hence, any pathway to extensive adoption of fuel-cell technology essentially includes some decrease in the amount of platinum needed. It could be done by exchanging it for some other catalyst material or by decreasing the amount of platinum required without compromising the performance.
A large amount of research has concentrated on the latter approach. Especially, scientists have concentrated on alloying platinum along with cobalt, actually diluting the amount of platinum needed to obtain a similar outcome.
The cause for this is that several platinum-cobalt alloys tend to exhibit a greater “active surface area”—the spaces present on the molecules of the catalyst where the appropriate chemical reactions can occur.
But fine-tuning the degree of alloying to obtain optimum ORR performance has been a great difficulty.
Professor Xiang synthesized a platinum-cobalt-carbon precursor (the compound that functions to produce a second compound, in this case, the platinum-cobalt alloy) by making use of dimethylamine borane (DMAB) as the reducing agent (a substance that grants electrons to another one in a similar chemical reaction).
This precursor was made to be heated to a high temperature in an environment of hydrogen and argon gas to generate a platinum-cobalt alloy that includes three platinum atoms to each cobalt one in the form of nano-scale particles.
The structure of the electrons in this particular platinum-cobalt alloy permits a high amount of activity on the membrane surface of the electrodes in the fuel cell. As a result, fuel cell performance is enhanced, and great stability for the fuel cell has been obtained.
This latter gain was illustrated by only mild performance deterioration following 10,000 cycles of the fuel cell. Additional testing inside single fuel cells displayed their approach was significantly surpassing the needs of US Department of Energy standards.
Having illustrated a decrease in the amount of platinum needed to obtain excellent PEMFC performance, Prof Xiang currently wishes to see if he could substitute the platinum-based catalyst in a complete manner. This could be done by using non-precious metals as the catalyst, again while retaining or enhancing performance and long-lasting stability.
Journal Reference:
Yang, B., et al. (2022) Secondary reduction strategy synthesis of Pt–Co nanoparticle catalysts towards enhanced the activity of proton exchange membrane fuel cells. Particuology. doi.org/10.1016/j.partic.2022.11.010.