Carbon dioxide (CO2) is a greenhouse gas produced when fossil fuels are used to produce energy. Using sunlight, researchers are striving to convert CO2 into valuable compounds or fuels.
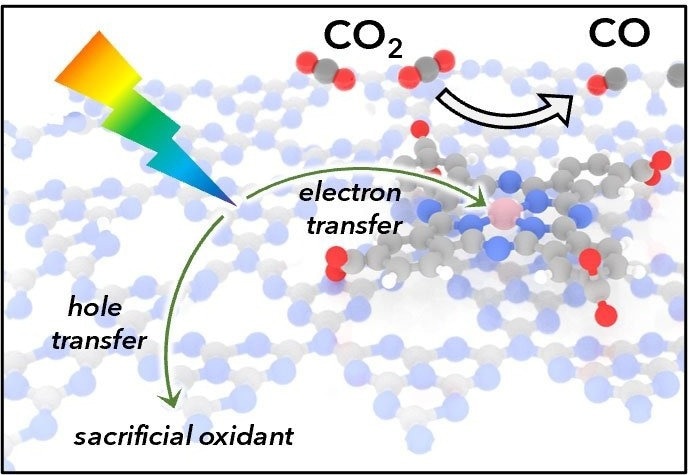
Depiction of light absorption by carbon nitride followed by electron transfer to a cobalt catalyst. The catalyst then reduces CO2 to CO. Acid-base interactions between the carbon nitride semiconductor and catalyst promote even coverage. Image Credit: Daniel Kurtz and Hailiang Wang.
Scientists have discovered a new hybrid material that combines a light-absorbing semiconductor with a cobalt catalyst to convert carbon dioxide into more usable carbon monoxide (CO).
To create this hybrid system, researchers needed to find a better approach to connect the catalyst to the material’s surface. They also investigated how the hybrid material behaves in light-driven chemistry. This will assist scientists in developing more effective and long-lasting systems for converting carbon dioxide into useful products.
The Impact
This research adds to current scientific attempts to discover novel ways to store energy. Converting CO2 to CO using sunlight could be a first step toward storing solar energy as a chemical fuel. This process could be used in conjunction with batteries and other energy storage devices.
Researchers must build rapid and efficient catalytic systems to make these solar chemical fuels realistic and viable. This research contributes to a better understanding of how light-absorbing hybrid systems can be used to drive the catalytic synthesis of chemical fuels utilizing solar energy.
Summary
Hybrid photocatalysis materials may combine the excellent selectivity and effectiveness of a molecular catalyst with the endurance of solid material support. This study, conducted at the University of North Carolina in Chapel Hill’s Center for Hybrid Approaches in Solar Energy to Liquid Fuels (CHASE), combines a known cobalt catalyst onto the surface of a light-absorbing carbon nitride material.
Scientists from Yale University, Emory University, and North Carolina State University were part of the study. The attachment strategy achieves a high surface coverage of the catalyst and uniformly organizes the molecules on the surface of the hybrid material. These two features are critical to the high catalytic performance of this hybrid material, which rapidly produces CO from CO2.
The development of a high-performance photocatalytic device is a big step forward. To build on this discovery, researchers must first comprehend the material's flaws and how it encourages reactivity.
These new studies both identify the active state of the molecular catalyst and investigate the process by which this active catalyst is formed, demonstrating that the active and highly reduced form of the catalyst takes some time to produce. Furthermore, this study associates a decline in catalyst efficiency with the breakdown of the catalyst itself, rather than the integrity of the entire hybrid system.
Funding
The study was solely supported as part of CHASE, an Energy Innovation Hub funded by the Department of Energy, Office of Science, and Office of Basic Energy Sciences. The Xe lamp utilized in this research was procured with internal funding from the Center for Research on Interface Structures and Phenomena at Yale University.
Journal Reference
Shang, B., et al. (2022) Monolayer Molecular Functionalization Enabled by Acid–Base Interaction for High-Performance Photochemical CO2 Reduction. ACS Energy Letters. doi.org/10.1021/acsenergylett.2c01147.