Scientists have succeeded in enhancing the specific capacity of Na3V2(PO4)3 (NVP), a novel electrode material, according to a recent study published in Advanced Science. Researchers also discovered its unusual extrinsic pseudocapacitance behavior.
 Origin of three-electron reaction mechanism and extrinsic pseudocapacitance behavior for the new NVP material. Image Credit: Hongyang Ma.
Origin of three-electron reaction mechanism and extrinsic pseudocapacitance behavior for the new NVP material. Image Credit: Hongyang Ma.
This research, directed by Prof. Bangchuan Zhao of the Chinese Academy of Sciences’ Hefei Institutes of Physical Science, efficiently activates the M1 site in the structure of NVP by lowering crystallinity and accomplishes the reversible insertion and removal of three sodium ions.
NVP has a hexagonal NASICON (or sodium super ion conductor) structure. Sodium ions occupy two disproportionate Wyckoff sites, with one-third at the 6b site (M1) and two-thirds at the 18e site (M2). From the standpoint of thermodynamic equilibrium and kinetics, sodium ions at the M1 site cannot participate in the redox reaction. During the charging and discharging processes, there is no electrochemical reactivity.
The insertion and removal of sodium ions are solely reversible at the M2 site. Through the V4+/V3+redox pair, only two sodium ions engage in the electrochemical reaction. As a result, NVP’s theoretical specific capacity is just 117.6 mAh g-1. The question of whether the specific capacity of NVP can be increased by activating the M1 site and exploiting the V5+/V4+ redox reaction is a major issue in this field.
In this research, the NVP precursor was electrostatically sprayed onto a carbon foam substrate, and the crystallinity of NVP was adjusted by varying the annealing temperature. NVP-E600 and NVP-E700 NVP materials were obtained. The annealing temperatures were 600 and 700 °C, respectively.
A coin-type cell was fabricated and its sodium storage performance was investigated using a nanocrystalline and amorphous phase coexisting structure NVP material as the cathode and a metal sodium sheet as the counter electrode.
The findings demonstrate that the nanocrystalline and amorphous phase coexisting structure of NVP material exhibited outstanding rate performance and cycle stability owing to the reversible de-insertion of three sodium ions. At 0.2 C (1 C = 117.6 mA g-1), it had a specific capacity of 179.6 mAh g-1 and a capacity retention rate of 99.6% after 200 cycles. It has a specific capacity of 73.5 mAh g-1 even at a high rate of 10 C.
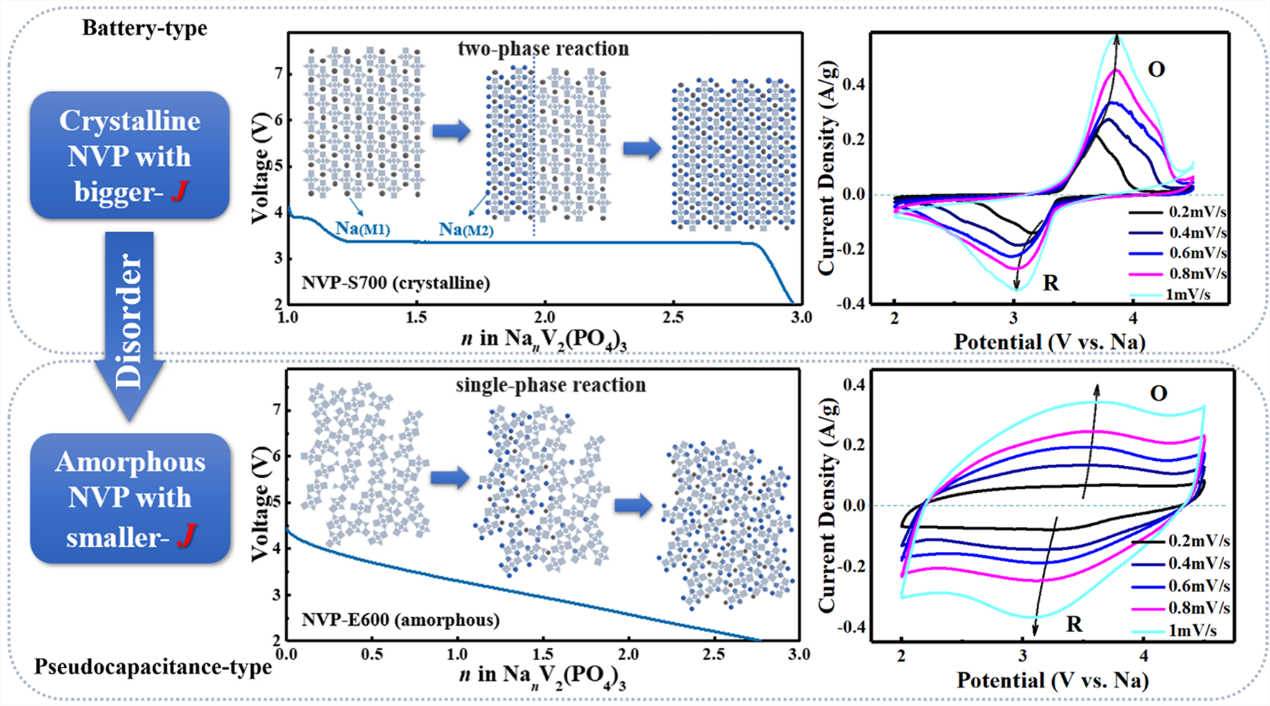
The electrochemical impedance spectrum and cyclic voltammetry curves reveal that this disordered NVP material has strong electrochemical reaction kinetics and that the charge storage was primarily pseudocapacitive, which differs significantly from the crystalline NVP with battery-type charge storage behavior.
The highly disordered material’s extrinsic pseudocapacitance behavior can be explained by the fact that the introduction of disordered structure changes the interaction between sodium ions in NVP materials, causing the charge-discharge process to transform from the original two-phase reaction to a single-phase reaction. This allows the platform in the charge-discharge curves and the rectangle in the cyclic voltammetry curves to disappear.
According to the researchers, this emphasizes the significance of the disordered structure for the three-electron reaction process and extrinsic pseudocapacitance in NVP cathodes of sodium-ion batteries.
Journal Reference:
Ma, H., et al. (2022) Crystallinity Tuning of Na3V2(PO4)3: Unlocking Sodium Storage Capacity and Inducing Pseudocapacitance Behavior. Advanced Science. doi.org/10.1002/advs.202203552.