A research team has enhanced the titanium oxo clusters’ capacity to absorb solar energy. Their work demonstrates a successful method for modifying the behaviors of these clusters in terms of light absorption by bringing in electron-rich heterometals. These findings could be used to improve solar energy conversion, which is currently constrained in certain ways.
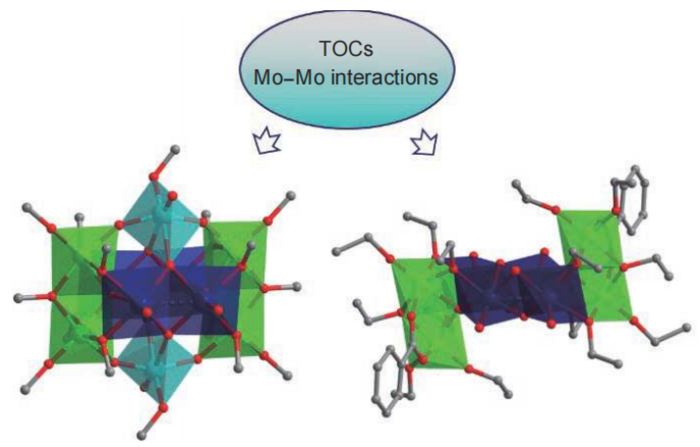 Polyhedral models showing the molecular structure of the two heterometallic clusters the research team created. Left model is TiIV4MoV4MoVI2O16(OCH3)16. Right model is TiIV4MoV4O10(OC2H5)14(C6H5COO)2. Image Credit: Polyoxometalates, Tsinghua University Press
Polyhedral models showing the molecular structure of the two heterometallic clusters the research team created. Left model is TiIV4MoV4MoVI2O16(OCH3)16. Right model is TiIV4MoV4O10(OC2H5)14(C6H5COO)2. Image Credit: Polyoxometalates, Tsinghua University Press
The team’s study was published on December 2nd, 2022, in the journal Polyoxometalates.
Solar energy has the potential to slow down the gradual depletion of the world’s energy supplies. The excellent stability, durability, and activity of titanium dioxide make it one of the most promising material candidates for solar conversion, according to scientists.
However, titanium dioxide has a wide bandgap, which means that only a small portion of the solar spectrum is used. Similarly, using metal doping techniques to increase impurity levels within prohibited bands is an excellent way to improve solar absorption and performance.
The team successfully built two heterometallic clusters to enhance the solar absorption and functionality of titanium oxo clusters. Any compound that has one or more metal atoms replaced by those from another metal is said to be heterometallic.
Single-crystal X-Ray diffraction analysis was used to identify heterometallic clusters and discovered that they all shared molybdenum interactions.
The introduction of electron-rich molybdenum (Mo) pairs as heterometals are primarily responsible for these structures’ enhanced visible-light absorption and significantly smaller optical band gaps, according to the team’s solid-state ultraviolet-visible absorption studies.
We discovered that the electron-rich Mo–Mo pairs could be introduced to titanium oxo clusters to enhance visible-light absorption.
Lei Zhang, Professor, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences
Functional molecular titanium dioxide materials are crystalline oxo clusters that can be tailored and have precise structures.
Most of the metals on the periodic table have been combined with titanium oxo clusters, where their electronic microscopic structure and performance can be altered. However, research on molybdenum-doped titanium oxo clusters has been scant.
Scientists are particularly interested in electron-rich metal oxo clusters with metal-metal bonds because of their potential applications as catalysts, electronics, and batteries. These clusters have distinct molecular and electronic structures. Band gap engineering and catalysis could benefit from clusters that have been altered by heterometals with delocalized electrons.
Molybdenum chloride was used by the team as a source of low-valent metals due to these factors. They played with the low-valent heterometallic molybdenum-titanium-oxo clusters in different arrangements while working with a carboxylic acid in alcohol media.
The team’s two heterometallic clusters share MoV-MoV bonds in all three directions. The simultaneous presence of MoV and MoVI in one of the clusters is revealed by the researchers’ X-Ray photoelectron spectroscopy measurements, indicating the first instance of mixed-valent MoV/VI doping in heterometallic titanium oxo clusters.
The potential applications of a semiconductor in optoelectronics are determined by its band gap. The team used diffuse reflectance spectroscopy analysis to look into the band gaps of their two heterometallic clusters.
They discovered that these two compounds’ optical band gaps are significantly smaller. They blame the introduction of electron-rich Mo-Mo pairs as heterometals for the reduction in band gaps.
The solid-state ultraviolet-visible spectroscopy measurements also revealed that the bands effectively shift toward the visible-light region, another discovery made by the team.
The team’s research not only increases the structural variety of metal oxo clusters but also offers a quick way to modify the properties of electron-rich titanium oxo clusters doped with molybdenum.
In the future, the team wants to create heterometallic oxo clusters with increased stability and visible-light absorption.
Zhang added, “The ultimate goal is to develop efficient cluster catalysts to split water into hydrogen using solar energy.”
The research team consists of Xiaofeng Yi, Jian Zhang, and Lei Zhang, from the Chinese Academy of Sciences’ Fujian Institute of Research on the Structure of Matter, along with Weizhou Chen, who works at Fuzhou University and the Chinese Academy of Sciences’ Fujian Institute of Research on the Structure of Matter.
The National Natural Science Foundation of China and the Natural Science Foundation of Fujian Province funded this study.
Journal Reference:
Chen, W., et al. (2023) Heterometallic Mo–Ti oxo clusters with metal–metal bonds: Preparation and visible-light absorption behaviors. Polyoxometalates. doi:10.26599/POM.2022.9140013.