One of the most effective methods for addressing climate change is CO2 hydrogenation, which combines green hydrogen with CO2 to solve three complex issues: high CO2 levels, the temporal mismatch between solar power production and demand, and hydrogen gas storage. The catalyst deactivates quickly because the CO2 hydrogenation process requires extremely high temperatures.
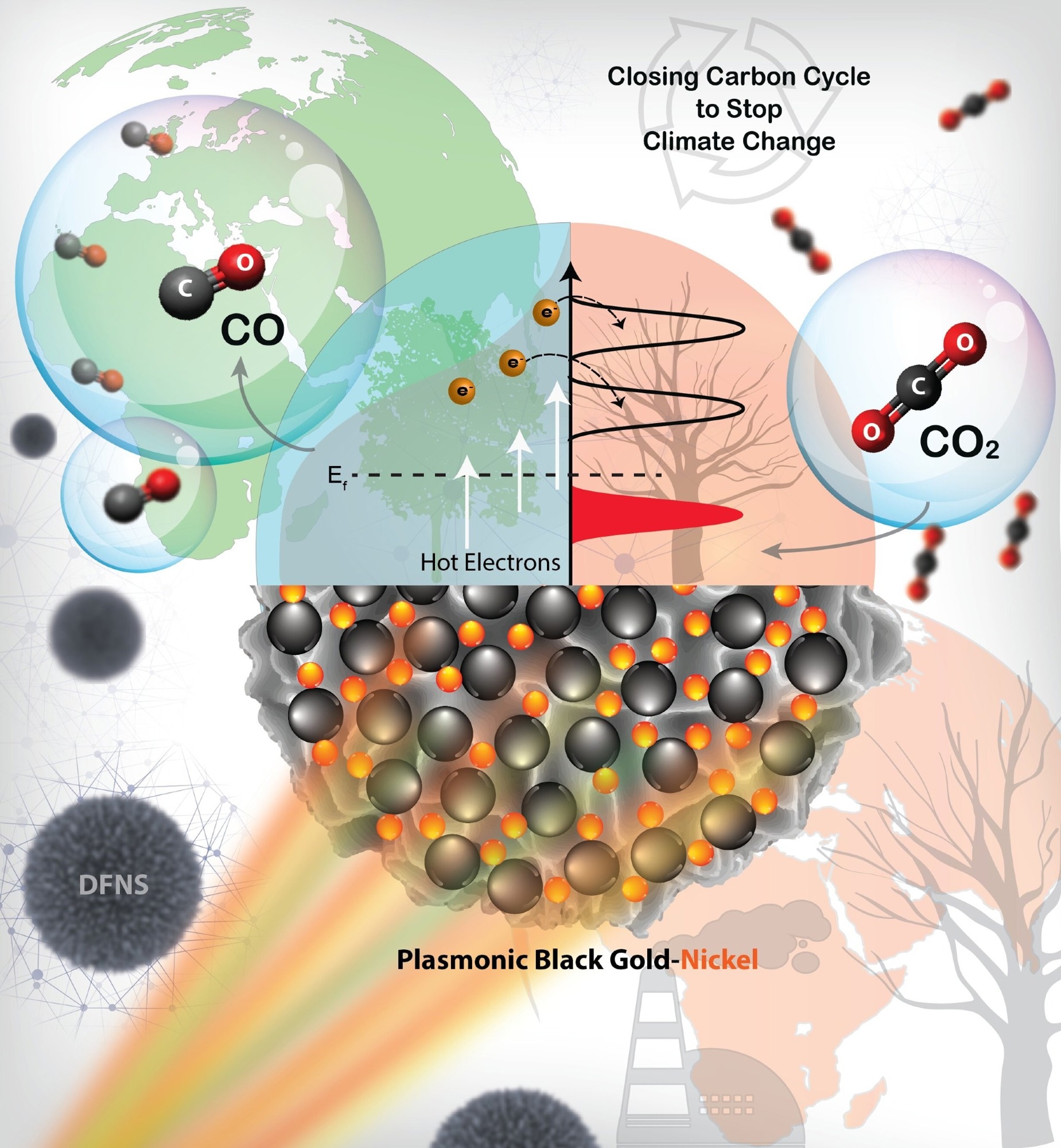 A plasmonic black gold nickel catalyzes strategically important CO2 hydrogenation reactions using solar energy, with an exceptional CO production rate. The reaction followed the hot-electron-mediated mechanism and provided a sustainable way to close the carbon cycle and thus combat climate change. Image Credit: Mr. Rishi Verma and Prof. Vivek Polshettiwar
A plasmonic black gold nickel catalyzes strategically important CO2 hydrogenation reactions using solar energy, with an exceptional CO production rate. The reaction followed the hot-electron-mediated mechanism and provided a sustainable way to close the carbon cycle and thus combat climate change. Image Credit: Mr. Rishi Verma and Prof. Vivek Polshettiwar
In this study, scientists at the Tata Institute of Fundamental Research (TIFR) in Mumbai investigated the possibility of catalyzing this high-temperature CO2 hydrogenation at a temperature between 0 and 10 °C by utilizing a plasmonic catalyst to excite both H2 and CO2. The team showed that plasmonic black gold-nickel effectively uses visible light to catalyze CO2 hydrogenation.
Without additional heating, the reaction occurred at temperatures ranging from 84 to 223 °C. The amount of catalytic activity was found to be doubled when compared to DPC-C4, and only DPC-C4-Ni showed measurable photoactivity.
In the flow circumstances, it demonstrated the best-reported CO production rate of 2464± 40 mmol gNi–1 h–1 and selectivity greater than 95%. The catalyst exhibited exceptionally high stability (100 hours).
The hot-electron mediated reaction mechanism was affirmed by the super-linear power law dependence on the light intensity (power law exponent of 5.6) with photocatalytic quantum efficiencies increasing with an increase in light intensity and reaction temperature, while the kinetic isotope effect (KIE) in light (1.91) was higher than in the dark.
The rapid electron injection from Au to Ni, which filled the Ni reactor with charge carriers, was demonstrated by ultrafast investigations of hot-carrier dynamics. The heated electron transfer from the gold to the nickel caused spectral signatures of such an indirect charge production, which was observed by the researchers.
Additionally, plasmon-induced high local field intensity augmentation was seen in DPC-C4-Ni in finite-difference time-domain simulations.
An in-situ DRIFTS investigation revealed that the generation of bridge carbonyl species was inhibited when the linearly bonded C=O vibrations on top of the Ni atom were stretched. By using linearly bonded nickel-CO, CO2 hydrogenation was accomplished directly. Therefore, CO desorption was effective, limiting hydrogenation to methane and producing CO selectivity of over 95%.
In addition to black gold’s superior light-harvesting abilities, the high production rate and selectivity were attributable to Ni NPs being extensively disseminated on black gold and offering a weakly bonded CO route.
Ni sites had excellent activity even at smaller particle sizes as a result of the nickel d-band electrons being excited to a higher energy level during the plasmonic damping of the black gold SPR and the hot electron transfer from the black gold to Ni filling the Ni d-band.
The exceptional catalytic performance of black gold-Ni could make it possible to create plasmonic catalysts for black gold-based catalytic processes such as CO2 reduction.
Journal Reference:
Verma, R., et al. (2023) Nickel-Laden Dendritic Plasmonic Colloidosomes of Black Gold: Forced Plasmon Mediated Photocatalytic CO2 Hydrogenation. ACS Nano. doi:10.1021/acsnano.2c10470.