With water as the only “waste” product, solid oxide fuel cells, often known as SOFCs, are a type of electrochemical device that uses hydrogen as fuel to produce electricity. Naturally, businesses and researchers have shown a great deal of interest in the development of SOFCs since there is a push to minimize human carbon production and lessen the effects of the climate crisis.
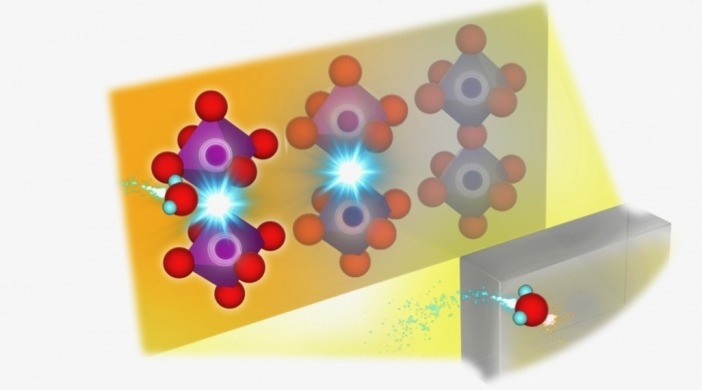 Probing where the protons go using synchrotron radiation. A computer rendering of the experiment. Using synchrotron radiation, and simulations via supercomputers and machine learning on top of thermogravimetric analysis, researchers were able to observe where protons are introduced in their perovskite based SOFC electrolyte. Image Credit: Kyushu University/Yamazaki Lab
Probing where the protons go using synchrotron radiation. A computer rendering of the experiment. Using synchrotron radiation, and simulations via supercomputers and machine learning on top of thermogravimetric analysis, researchers were able to observe where protons are introduced in their perovskite based SOFC electrolyte. Image Credit: Kyushu University/Yamazaki Lab
A research team led by Kyushu University has discovered the chemical mechanisms of a perovskite-based electrolyte they designed for SOFCs, which could accelerate the production of more effective SOFCs.
The scientists used a combination of thermogravimetric analysis, machine learning, large-scale simulations, and synchrotron radiation analysis to identify the location of the active site, which is where hydrogen atoms are incorporated into the perovskite lattice with the objective of generating energy. The findings were released in the Chemistry of Materials journal.
At its most fundamental level, a fuel cell is just a machine that produces electricity by assisting the splitting of a hydrogen atom into its positively and negatively charged proton and electron. The electron is utilized to generate electricity and then combines with a proton and oxygen and produces water as a “waste” product.
The electrolyte is the actual material that drives everything. The material functions as an atomic sieve to help move certain atoms across the fuel cell. Those atoms could represent protons or oxygen, depending on the type of fuel cell.
Although many people may not be familiar with the name SOFCs, the technology has already found commercial use in generators for single-family houses. Despite this, they continue to be costly, with their high operating temperature being one of the biggest challenges.
Conventional SOFCs need to be at 700–1000 ℃ for the electrolyte to perform efficiently. Naturally, there is a global race to develop SOFC electrolytes that can operate at lower temperatures of around 300–450 ℃. One such promising materials are perovskites.
Yoshihiro Yamazaki, Study Lead and Professor, Platform of Inter/Transdisciplinary Energy Research, Kyushu University
Perovskites are a class of materials with a particular crystalline structure that enables them to have distinctive physical, optical, and even electrical capabilities. Additionally, a significant amount of research focuses on creating and evaluating an almost endless variety of potential perovskites since they can be synthetically created using various atoms.
Creating improved SOFC electrolytes is one such example.
Yamazaki added, “In our past work we developed a Barium and Zirconium based perovskite with the chemical composition BaZrO3. By replacing the Zr site with a high concentration of Scandium, or Sc, we succeeded in making a high-performance electrolyte that can function at our target temperature of 40"0 ℃.”
“Of course, that was only a part of what we wanted to find. We also were investigating a question that hadn't been solved for over three decades: where in the electrolyte's lattice do the protons get introduced?” he continued.
The high operating temperature and fluctuating pressure from the water, the fuel cell’s source of hydrogen, made it challenging to investigate the inner workings of SOFCs.
To move past these problems, the researchers used synchrotron radiation—the electromagnetic radiation released by particle accelerators—to perform X-Ray absorption spectroscopy studies on their perovskite electrolyte while the fuel cell was operating at around 400 °C.
Yamazaki further stated, “These results gave us insight into where in the material's chemical structure the protons would be incorporated. From there we applied machine learning, and using a supercomputer calculated possible structural configurations of the material. By carefully comparing the predicted results with experimental data we were able to clarify the structural changes the electrolyte undertakes when active.”
He concluded, “Now that we have the fundamental innerworkings of the electrolyte we can being optimizing its nanostructures and even propose new materials that can lead to more efficient fuel cells, and even ones that work at wider temperature ranges.”
Journal Reference:
Hoshino, K., et al. (2023) Probing Local Environments of Oxygen Vacancies Responsible for Hydration in Sc-Doped Barium Zirconates at Elevated Temperatures: In Situ X-ray Absorption Spectroscopy, Thermogravimetry, and Active Learning Ab Initio Replica Exchange Monte Carlo Simulations. Chemistry of Materials. doi:10.1021/acs.chemmater.2c02116.