Methane makes up 99.5% of the combustible ice, making it a vital reserve energy source. The reserves of combustible ice in the South China Sea are estimated to be at least 80 billion tons of oil equivalent.
 The schematic of the photocatalytic methane halogenation. Image Credit: Jun Ma et al.
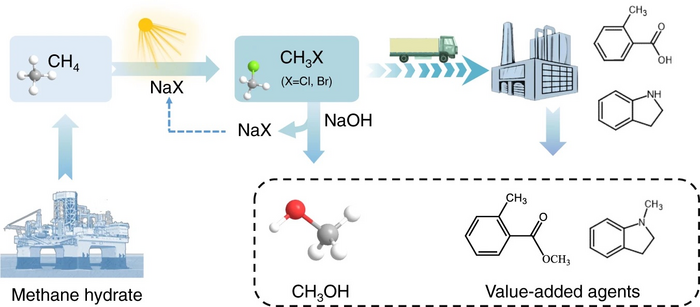
The schematic of the photocatalytic methane halogenation. Image Credit: Jun Ma et al.
The current mining procedure will result in the gasification of combustible ice during the decompression phase, posing a significant barrier to methane gas storage and transportation. If methane can be turned into liquid products under offshore conditions, it will open up new possibilities for the use of combustible ice.
As a result, a group headed by Prof. Yujie Xiong and Prof. Ran Long from the University of Science and Technology (USTC) developed an effective photocatalytic methane halogenation technology that uses only light, methane, and seawater, the products of which can be converted into methanol and pharmaceutical intermediates. On March 14th, 2023, the study was published in Nature Communications.
Methyl halides are frequently employed in manufacturing high-value-added chemicals and fuels like methanol, acetic acid, and propylene as a type of adaptable platform molecule. However, conventional methyl halide synthesis typically entails corrosive feedstocks like chlorine and hydrogen bromide, as well as severe reaction conditions, necessitating sophisticated methods and massive energy consumption and posing significant harm to the environment.
The researchers developed copper-doped titania catalysis and employed widely available alkali halides as halogenation agents to achieve an effective methyl halide synthesis under light, with a production rate of 1 mmol h-1m-2.
This approach uses offshore light and seawater conditions to efficiently convert methane to chloromethane, proving the viability of photocatalytic methane chlorination technology in the use of combustible ice. The researchers developed a tandem reaction device based on this approach to synthesize methanol and pharmaceutical intermediates with methane.
This discovery paved the way for the use of combustible ice and the conversion of methane to high-value-added chemical products.
Journal Reference:
Ma, J., et al. (2023). Sustainable methane utilization technology via photocatalytic halogenation with alkali halides. Nature Communications. doi.org/10.1038/s41467-023-36977-0.