According to the University of Illinois Urbana-Champaign investigators, newly developed “smart” coatings for surgical orthopedic implants can detect strain on the devices to provide early warning of implant failures while destroying infection-causing bacteria. The coatings combine flexible sensors with a nanostructured antibacterial surface inspired by dragonfly and cicadas’ wings.
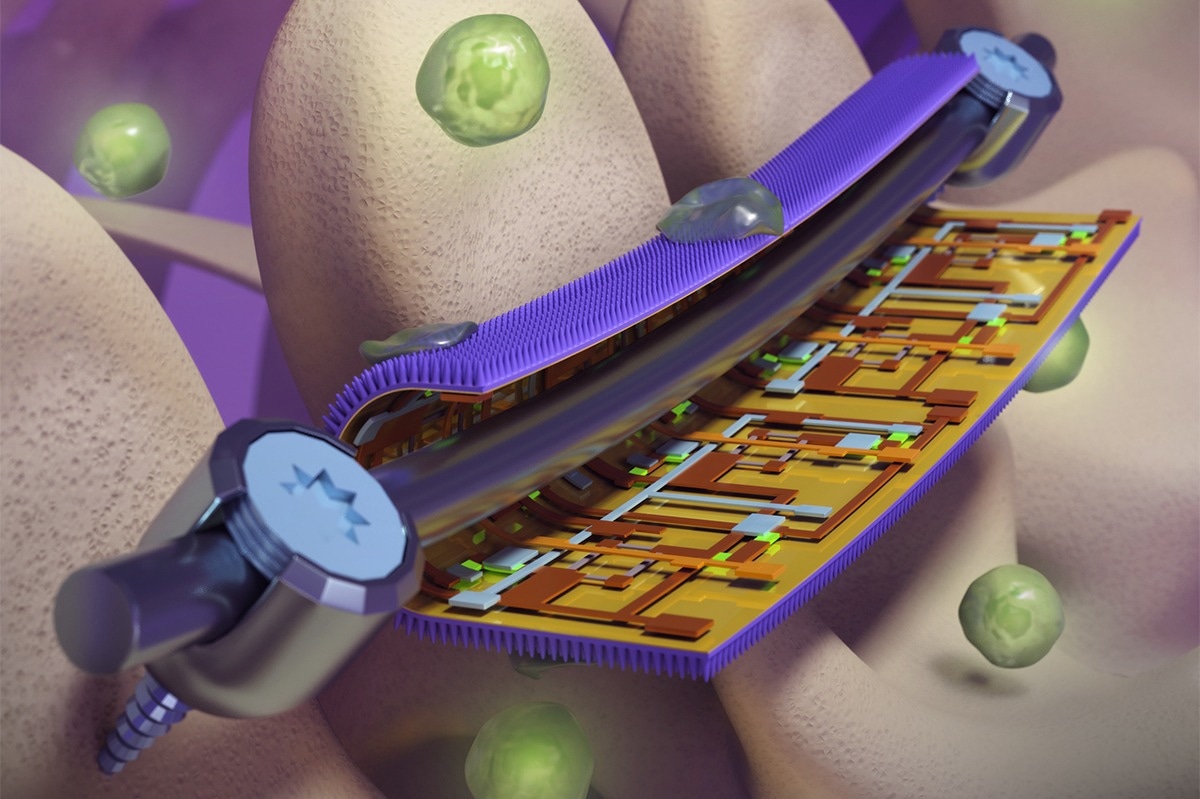 Smart coatings on orthopedic implants, developed at the University of Illinois Urbana-Champaign, has bacteria-killing nanopillars on one side and strain-mapping flexible electronics on the other. This could help physicians guide patient rehabilitation and repair or replace devices before they fail. Image Credit: Beckman Imaging Technology Group.
Smart coatings on orthopedic implants, developed at the University of Illinois Urbana-Champaign, has bacteria-killing nanopillars on one side and strain-mapping flexible electronics on the other. This could help physicians guide patient rehabilitation and repair or replace devices before they fail. Image Credit: Beckman Imaging Technology Group.
A multidisciplinary team of researchers discovered the coatings prevented infection in live mice and mapped strain in commercial implants placed on sheep spines to warn of potential implant or healing failures in a new study published in the journal Science Advances.
This is a combination of bio-inspired nanomaterial design with flexible electronics to battle a complicated, long-term biomedical problem.
Qing Cao, Study Leader and Professor, Materials Science and Engineering, University of Illinois Urbana-Champaign
Infection and device failure are common issues with orthopedic implants, affecting up to 10% of patients, according to Cao. Several ways to fight infection have been tried. However, all have serious limits, according to him: Biofilms can still form on water-repellent surfaces, and coatings packed with antibiotic chemicals or drugs degrade with time, causing harmful effects on surrounding tissue and having minimal efficacy against drug-resistant strains of bacterial pathogens.
The Illinois team was inspired by the naturally antibacterial wings of cicadas and dragonflies and manufactured a thin foil patterned with nanoscale pillars similar to those seen on the insects’ wings. When a bacterial cell tries to adhere to the foil, the pillars puncture its cell wall and kill it.
Using a mechanical approach to killing bacteria allowed us to bypass a lot of the problems with chemical approaches, while still giving us the flexibility needed to apply the coating to implant surfaces.
Gee Lau, Study Co-Author and Professor, Pathobiology, University of Illinois Urbana-Champaign
The researchers inserted arrays of highly sensitive, flexible electronic sensors to detect strain on the reverse side of the nanostructured foil, where it contacts the implant device. According to the researchers, this might allow clinicians to monitor individual patients’ healing status, direct their rehabilitation to cut recovery time and minimize hazards, and repair or replace equipment before they fail.
The engineering team then collaborated with veterinary clinical medicine Professor Annette McCoy to put their prototype devices to the test. They implanted the foils in live mice and watched them for any signs of infection, even after introducing bacteria.
The team also put the coatings on commercially available spinal implants and measured implant strain in sheep spines under normal load to diagnose device failure. Both purposes were efficiently served by the coatings.
The prototype electronics required wires, but the researchers intend to develop wireless power and data communications interfaces for their coatings next, which will be a critical step toward clinical application, according to Cao. The scientists are also aiming to scale up the production of the nanopillar-textured bacteria-killing foil.
“These types of antibacterial coatings have a lot of potential applications, and since ours uses a mechanical mechanism, it has potential for places where chemicals or heavy metal ions – as are used in commercial antimicrobial coatings now – would be detrimental,” Cao notes.
The study was supported by the National Science Foundation and the US Congressionally Directed Medical Research Programs.
Journal Reference:
Zhang, Y., et al. (2023). A smart coating with integrated physical antimicrobial and strain-mapping functionalities for orthopedic implants. Science Advances. doi.org/10.1126/sciadv.adg7397.