Recent research guided by a team from the University of Oxford may bring improved electric vehicle (EV) batteries one step closer. The mechanisms that cause lithium metal solid-state batteries (Li-SSBs) to degrade were discovered using improved imaging techniques.
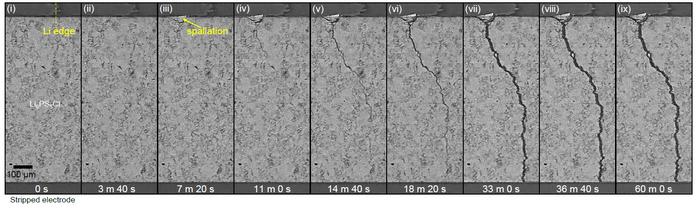 X-Ray computed tomography images showing the progressive growth of a lithium dendrite crack within a solid-state battery during the charging process. Image credit: Dominic Melvin, Nature, 2023.
X-Ray computed tomography images showing the progressive growth of a lithium dendrite crack within a solid-state battery during the charging process. Image credit: Dominic Melvin, Nature, 2023.
If these challenges can be addressed, solid-state batteries with lithium metal anodes could significantly increase EV battery range, safety, and performance, as well as aid advance electrically powered aviation. The study was published in the journal Nature.
Progressing solid-state batteries with lithium metal anodes is one of the most important challenges facing the advancement of battery technologies. While lithium-ion batteries of today will continue to improve, research into solid-state batteries has the potential to be high-reward and a gamechanger technology.
Dominic Melvin, Study Co-Lead Authors and PhD Student, Department of Materials, University of Oxford
Li-SSBs differ from other batteries in that they employ a solid electrolyte instead of the flammable liquid electrolyte found in ordinary batteries and use lithium metal as the anode (negative electrode). The use of a solid electrolyte enhances safety, and the use of lithium metal allows for additional energy storage.
A significant issue with Li-SSBs is that they are prone to short-circuiting when charging due to the formation of “dendrites,” which are filaments of lithium metal that crack through the ceramic electrolyte.
Scientists from the University of Oxford’s Departments of Materials, Chemistry, and Engineering Science have led a series of in-depth examinations as part of the Faraday Institution’s SOLBAT project to learn more about how this short-circuiting occurs.
Researchers at Diamond Light Source used an advanced imaging technology called X-Ray computed tomography to observe dendrite failure in unprecedented detail throughout the charging process. The new imaging study showed that dendrite crack initiation and propagation are distinct processes powered by unique underlying mechanisms.
When lithium collects in subsurface pores, dendrite cracks form. When the pores become clogged, additional charging of the battery raises the pressure, causing breaking. In contrast, propagation takes place with lithium only partly filling the crack, through a wedge-opening mechanism that forces the crack open from the rear.
This novel understanding guides the way forward to overcome the technological challenges of Li-SSBs.
For instance, while pressure at the lithium anode can be good to avoid gaps developing at the interface with the solid electrolyte on discharge, our results demonstrate that too much pressure can be detrimental, making dendrite propagation and short-circuit on charging more likely.
Dominic Melvin, Study Co-Lead Authors and PhD Student, Department of Materials, University of Oxford
The process by which a soft metal such as lithium can penetrate a highly dense hard ceramic electrolyte has proved challenging to understand with many important contributions by excellent scientists around the world. We hope the additional insights we have gained will help the progress of solid-state battery research towards a practical device.
Sir Peter Bruce, Wolfson Chair and Professor, Materials, University of Oxford
Sir Peter Bruce is also the Chief Scientist of the Faraday Institution, and corresponding author of the study.
Faraday Institution’s recent report states that SSBs might satisfy 50% of the global demand for batteries in consumer electronics, over 10% in aircraft, and 30% in transportation by 2040.
Professor Pam Thomas, CEO, Faraday Institution, notes, “SOLBAT researchers continue to develop a mechanistic understanding of solid-state battery failure—one hurdle that needs to be overcome before high-power batteries with commercially relevant performance could be realized for automotive applications. The project is informing strategies that cell manufacturers might use to avoid cell failure for this technology. This application-inspired research is a prime example of the type of scientific advances that the Faraday Institution was set up to drive.”
Journal Reference:
Ning, Z., et al. (2023). Dendrite initiation and propagation in lithium metal solid-state batteries. Nature. doi.org/10.1038/s41586-023-05970-4.