Radboud University researchers have created synthetic molecules that mimic organic molecules. Researchers Alex Khajetoorians and Daniel Wegner have formed a team to imitate the behavior of natural molecules using synthetic compounds.
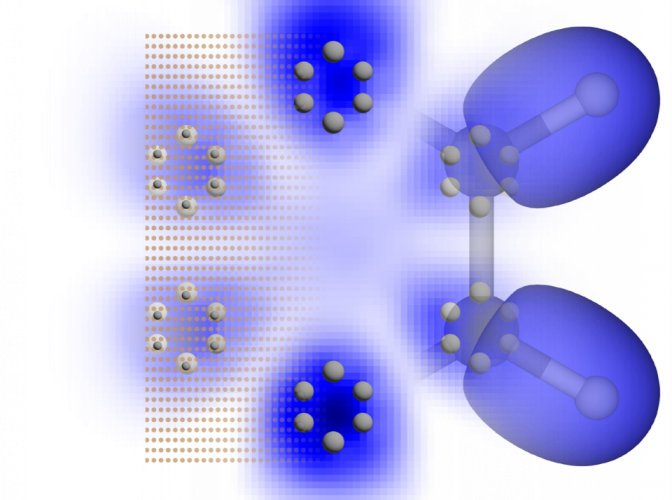
Image Credit: Radboud University
They can alter a molecule’s characteristics in ways that are often challenging or unfeasible, and they will have a far greater understanding of how molecules evolve as a result.
A few years ago, we had this crazy idea to build a quantum simulator. We wanted to create artificial molecules that resembled real molecules. So, we developed a system in which we trapped electrons. Electrons surround a molecule like a cloud, and we used those trapped electrons to build an artificial molecule.
Emil Sierda, Postdoctoral Researcher, Faculty of Science, Radboud University
The team’s findings were astounding.
Sierda added, “The resemblance between what we built and real molecules was uncanny.”
Changing Molecules
Making molecules is difficult enough. What is often harder, is to understand how certain molecules react, for example how they change when they are twisted or altered.
Alex Khajetoorians, Head, Scanning Probe Microscopy (SPM) Department, Radboud University
Chemistry is based on how molecules change and interact, which causes chemical reactions like the synthesis of water from hydrogen and oxygen.
Khajetoorians stated, “We wanted to simulate molecules, so we could have the ultimate toolkit to bend them and tune them in ways that are nearly impossible with real molecules. In that way we can say something about real molecules, without making them, or without having to deal with the challenges they present, like their constantly changing shape.”
Benzene
The researchers simulated benzene, one of the fundamental organic molecules in chemistry, using their simulator. Styrene, which is used to create polystyrene, is one of the many molecules that benzene serves as a base compound.
Khajetoorians further stated, “By making benzene, we simulated a textbook organic molecule, and built a molecule that is made up of elements that are not organic.”
Additionally, the molecules are ten times larger than their actual counterparts, making them simpler to control.
Practical Uses
This new method has countless applications.
We have only begun to imagine what we can use this for. We have so many ideas that it is hard to decide where to start.
Daniel Wegner, Assistant Professor, Scanning Probe Microscopy (SPM) Department, Radboud University
The simulator will benefit researchers in every aspect of science by helping them better comprehend molecules and their reactions.
Wegner added, “New materials for future computer hardware are really hard to make, for instance. By making a simulated version, we can look for the novel properties and functionalities of certain molecules and evaluate whether it will be worth making the real material.”
Everything from creating artificial single-molecule electronic gadgets to deciphering chemical reactions in slow-motion videos to reducing the number of transistors on computer chips may be feasible in the long future. Even the use of quantum simulators as quantum computers has been proposed.
Sierda concluded, “But that is a long way to go, for now we can start by beginning to understand molecules in a way we never understood before.”
Radboud University researchers Malte Rösner (Theory of Condensed Matter), Mikhail Katsnelson (Theory of Condensed Matter), Gerrit Groenenboom (Theoretical Chemistry), Daniel Wegner (SPM), and Alex Khajetoorians (SPM) collaborated on the study.
Journal Reference
Sierda, E., et al. (2023) Quantum simulator to emulate lower-dimensional molecular structure. Science. doi:10.1126/science.adf2685