Solid-state batteries, as opposed to the more popularly recognized lithium-ion batteries, use solid electrodes and solid electrolytes. Solid-state batteries eliminate several disadvantages of liquid-based batteries, including flammability, low voltage, unstable reactants, and poor long-term cyclability and strength.
All-solid-state rechargeable air battery with redox-active organic negative electrode. The battery, which uses a polymeric dihydroxy-benzoquinone-based negative electrode and a Nafion-based solid electrolyte, exhibits high Coulombic efficiency and discharge capacity. Image Credit: Waseda University
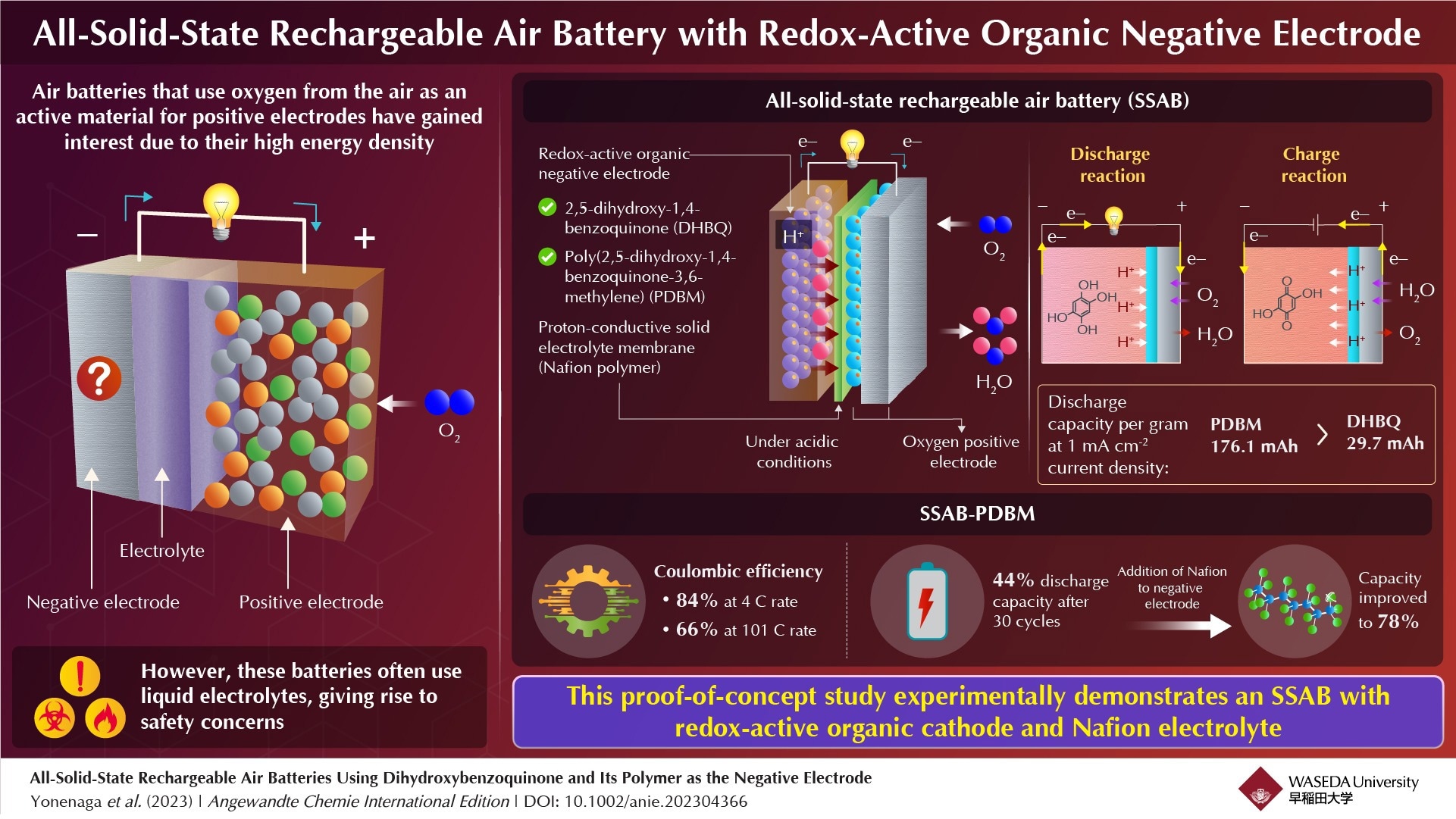
Researchers have recently created an all-solid-state rechargeable air battery made up of a redox-active organic negative electrode and a proton-conductive polymer electrolyte.
Negative electrodes in batteries are frequently made of active materials like metals. In recently developed rechargeable metal-air batteries with oxygen-reducing positive electrodes, redox-active organic molecules, such as quinone- and amine-based molecules, have been employed as negative electrodes. The redox reactions taking place here involve protons and hydroxide ions.
These batteries operate well and are almost at their theoretical maximum capacity. Furthermore, using redox-active organic molecules in rechargeable air batteries eliminates issues with metals, such as the development of “dendrites,” which have an adverse effect on battery performance and the environment.
These batteries, like metal-based batteries, use liquid electrolytes, which raise serious safety issues due to their high electrical resistance, leaching effects, and flammability.
Now, a team of Japanese researchers has created an all-solid-state rechargeable air battery (SSAB) and examined its capacity and endurance in a new study that was published in Angewandte Chemie International Edition on May 2nd, 2023.
Professor Kenji Miyatake of Waseda University and the University of Yamanashi executed the study, which was co-authored by Professor Kenichi Oyaizu of Waseda University.
Due to their stable and reversible redox reactions in acidic environments, the researchers selected the chemical 2,5-dihydroxy-1,4-benzoquinone (DHBQ) and its polymer poly(2,5-dihydroxy-1,4-benzoquinone-3,6-methylene) (PDBM) as active materials for the negative electrode.
They also used Nafion, a proton-conductive polymer, as the solid electrolyte in place of traditional liquid electrolytes.
To the best of my knowledge, no air batteries based on organic electrodes and solid polymer electrolyte have been developed yet.
Kenji Miyatake, Professor, Waseda University
After the SSAB was installed, the researchers experimentally evaluated its cyclability, rate characteristics, and charge-discharge performance. They discovered that the SSAB does not degrade in the presence of water and oxygen, in contrast to conventional air batteries, which use a metallic negative electrode and an organic liquid electrolyte.
Furthermore, substituting the redox-active molecule DHBQ for its polymeric equivalent PDBM resulted in a more effective negative electrode. At a constant current density of 1 mAcm–2, the SSAB-DHBQ had a per-gram-discharge capacity of 29.7 mAh, whereas the SSAB-PDBM had a capacity of 176.1 mAh.
The coulombic efficiency of SSAB-PDBM was 84% at a 4 C rate and subsequently declined to 66% at a 101 C rate, according to the researchers. While the discharge capacity of SSAB-PDBM decreased to 44% after 30 cycles, the researchers were able to dramatically boost it to 78% by increasing the proton-conductive polymer content of the negative electrode.
The inclusion of Nafion increased the performance and durability of the PDBM-based electrode based on the electron microscopic images.
This research shows that an SSAB with redox-active organic molecules as the negative electrode, a proton-conductive polymer as the solid electrolyte, and an oxygen-reducing, diffusion-type positive electrode can work successfully. The researchers think that this will pave the way for additional advancements.
Miyatake concluded, “This technology can extend the battery life of small electronic gadgets such as smartphones and eventually contribute to realizing a carbon-free society.”
The Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan funded this research in part through Grants-in-Aid for Scientific Research (18H05515, 23H02058), the MEXT Program: Data Creation and Utilization Type Material Research and Development Project (JPMXP1122712807), and JKA promotion funds from AUTORACE.
Journal Reference
Yonenaga, M., et al. (2023) All-Solid-State Rechargeable Air Batteries Using Dihydroxybenzoquinone and Its Polymer as the Negative Electrode. Angewandte Chemie International Edition. doi:10.1002/anie.202304366.