Dr. Minah Lee of the Energy Storage Research Center at the Korea Advanced Institute of Science and Technology (KIST) led a research team that created a chemical activation strategy for magnesium metal that allows for the efficient operation of magnesium batteries in common electrolytes that are free of corrosive additives and can be mass-produced.
Comparison of electrochemical reversibility of magnesium metal before and after chemical activation. Image Credit: Korea Institute of Science and Technology
While the need for lithium-ion batteries is skyrocketing due to the rapid increase of the electric vehicle and energy storage system (ESS) markets, the supply and demand for their raw materials, like lithium and cobalt, are heavily reliant on specific countries, raising concerns about ensuring a stable supply chain.
As a result, study on next-generation secondary batteries has been active, and secondary batteries utilizing magnesium, which is rich in the earth's crust, are gaining interest.
Since secondary magnesium batteries use Mg2+, a divalent ion, rather than monovalent alkali metal ions like lithium, they can have a high energy density. The highest energy density can be produced by directly using magnesium metal as an anode, which has a volumetric capacity of approximately 1.9 times that of lithium metal.
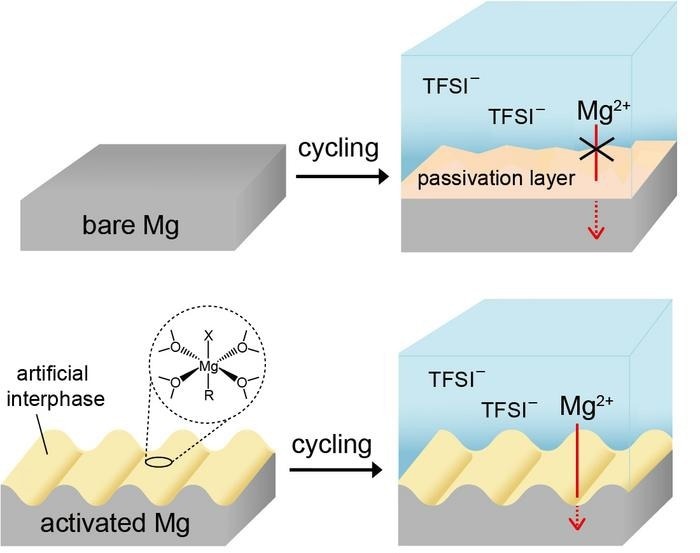
Even with these benefits, the difficulties of charging and discharging magnesium metal effectively due to its reactivity with electrolytes has hampered its commercialization. KIST scientists have devised a method for inducing a highly efficient charge and discharge reaction in magnesium metal, potentially paving the way for the commercialization of secondary magnesium batteries.
Unlike earlier studies that used corrosive electrolytes to expedite magnesium charging and discharging, the investigators used a common electrolyte with a similar composition to existing commercial electrolytes, allowing the use of high-voltage electrodes while minimizing the corrosion of battery components.
The researchers created an artificial protective layer on the magnesium surface with a unique composition based on magnesium alkyl halide oligomers using a simple procedure of dipping the magnesium metal to be used as the anode into a reactive alkyl halide solution prior to cell assembly.
They discovered that using a specific reaction fluid aided in the creation of nanostructures on the magnesium surface, which aided in the dissolving and deposition of magnesium. Based on this, they suppressed undesired electrolyte reactions and increased reaction area using nanostructuring to produce extremely efficient magnesium cycling.
While charging and discharging magnesium metal in a common electrolyte without corrosive additives, the overpotential may be decreased from more than 2 V to less than 0.2 V using the proposed technology, and the Coulombic efficiency can be raised from less than 10% to more than 99.5%.
The team demonstrated steady charging and discharging of activated magnesium metal for 990 cycles, demonstrating that magnesium rechargeable batteries can function in mass-produced electrolytes.
This work provides a new direction for the existing magnesium secondary battery research, which has been using corrosive electrolytes that prevent the formation of interfacial layers on magnesium metal surfaces. It will increase the possibility of low-cost, high-energy-density magnesium secondary batteries based on common electrolytes suitable for energy storage systems (ESS).
Dr. Minah Lee, Energy Storage Research Center, Korea Advanced Institute of Science and Technology
Journal Reference
Jeon, A-Re., et al. (2023). Reversible Magnesium Metal Cycling in Additive-Free Simple Salt Electrolytes Enabled by Spontaneous Chemical Activation. ACS Nano. doi.org/10.1021/acsnano.2c08672.