A global study team has employed theoretical predictions to determine and then make a new electrocatalyst that considerably helps the oxygen evolution reaction (OER).
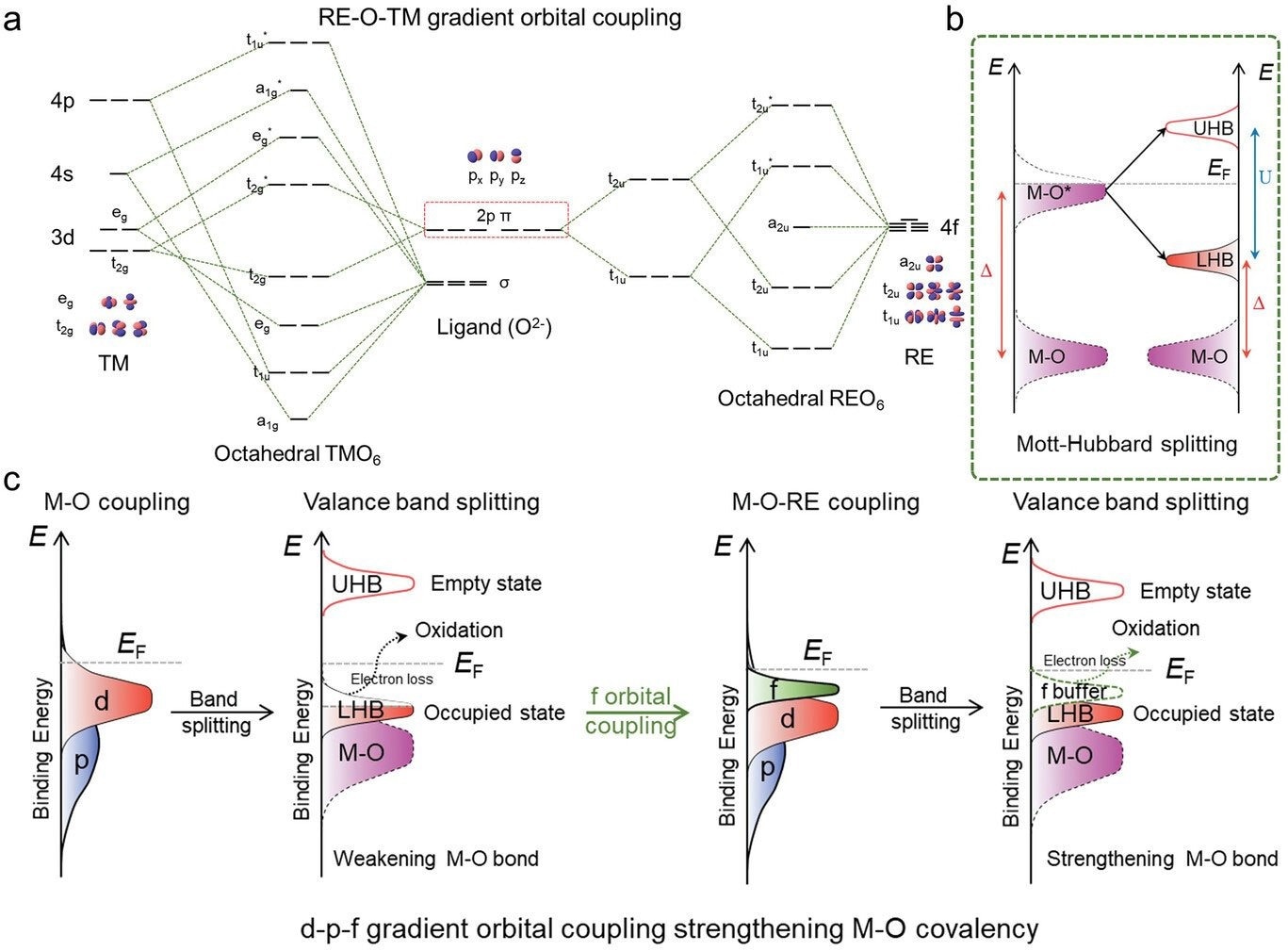 The proposed theoretical mechanism for the rationality in the gradient orbital coupling of TM-O-RE unit site. Image Credit: Hao Li et al.
The proposed theoretical mechanism for the rationality in the gradient orbital coupling of TM-O-RE unit site. Image Credit: Hao Li et al.
Details of their results were published in the journal Advanced Materials.
Electrolysis is harnessed by water-splitting electrolyzers to uncouple hydrogen from oxygen. The produced hydrogen gas can be utilized as a clean fuel, while the oxygen gas can be used for industrial processes, medical purposes, or just released into the atmosphere.
Water molecules are oxidized at the anode in OER. It has established itself to be a hindrance to the broader application of these devices. The OER results in energy loss and needs additional voltage to influence the reaction (overpotential).
To avoid this, researchers are seeking rare-earth-based transition metal oxides (RE-TMO), which aid in attaining a more successful OER process. However, these rely on costly and scarce metals, further restricting their industrial application. Furthermore, much stays mysterious about the way they function.
The predictive power of theory helped us overcome this shortcoming. Given that finding effective and low-cost OER catalysts requires a lot of trial-and-error, we used theory to predict that doping cerium (Ce) into cobalt oxides (CoO) would lead to a better performing and more stable electrocatalyst.
Hao Li, Study Corresponding Author and Associate Professor, Advanced Institute for Materials Research, Tohoku University
And the predictions of the team are proven true. With the use of a special plasma technique, they integrated cerium with cobalt oxide prior to conducting tests on the material. The outcomes verified the promising performance, which had an overpotential of just 261 mV at 10 mA cm−2 and powerful electrochemical stability, higher than individual CoO.
In-situ electrochemical Raman spectroscopy and X-Ray absorption spectroscopy showed that the cerium atoms made the cobalt oxide more robust by altering how the atoms are connected. The new atoms’ arrangement resulted in a more effective OER.
Li and his group are confident that their discovery can result in more developments in making more successful RE-TMOs. “We believe our Ce-CoO model can serve as a basis for the mechanistic understanding and structural design of high-performance RE-TMO catalysts,” they concluded.
Journal Reference:
Li, M., et al. (2023). Reinforce the Co‐O Covalency via Ce(4 f )‐O(2 p )‐Co(3 d ) Gradient Orbital Coupling for High‐efficiency Oxygen Evolution. Advanced Materials. doi.org/10.1002/adma.202302462.