At the Department of Energy’s Oak Ridge National Laboratory (ORNL), scientists have successfully used neutron reflectometry to look inside a working solid-state battery and track its electrochemistry for the first time.
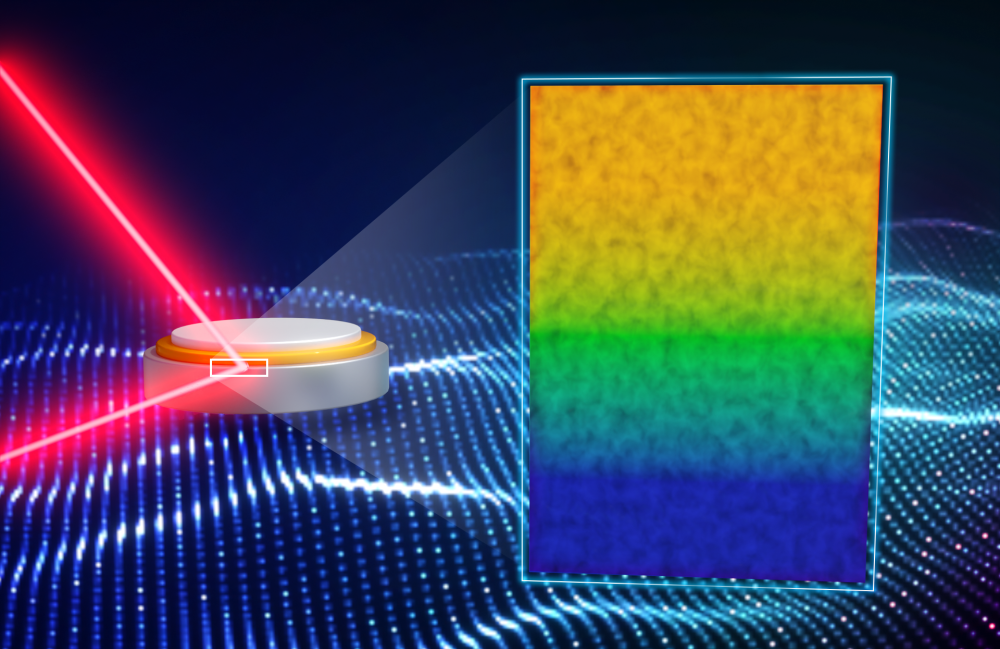
In a solid-state battery, reactive lithium metal (blue) can coexist stably with a solid electrolyte called LiPON (yellow) when an interphase (green), about 70 atoms thick, forms. Image Credit: Jill Hemman/ORNL, U.S. Dept. of Energy.
The researchers discovered that its outstanding performance was all down to an extremely thin layer, across which charged lithium atoms quickly flow as they move from anode to cathode and blend into a solid electrolyte.
“We want better batteries. That means more energy density, lower cost, faster and safer battery charging and longer life,” stated ORNL’s Andrew Westover, who co-led a study reported in ACS Energy Letters with James Browning at the lab’s Spallation Neutron Source.
Rechargeable batteries are reliant on lithium, a small metal atom that packs tightly into the negatively charged anode to enhance energy density. However, lithium is unstable with most electrolytes — a factor in the flammability of laptop, smartphone, and electric vehicle batteries that use liquid electrolytes.
Westover stated, “To fix the flammability issue, we want to switch to solid electrolytes.”
This is where lithium phosphorus oxynitride, otherwise known as LiPON - a solid electrolyte invented at ORNL almost three decades ago - comes into play.
It’s never been understood why it works really well. We want to make what works with LiPON work on a much larger scale. But we have to understand it first.
Andrew Westover, Study Co-Led Author, Oak Ridge National Laboratory
Previous work has shown that the solid electrolyte interphase, or SEI — a layer that protects and stabilizes the solid-state battery — is a major contributor to its repeated charge and discharge capabilities. In this case, the interphase is a chemical gradient that is made up of a lithium-rich layer whose lithium content decreases as it blends into pure LiPON.
Browning stated, “In a normal battery, an interphase forms between the electrolyte and the working electrode. Over time as you cycle a battery—charge and discharge it— that material can change in composition and thickness.”
If you have a good SEI, your battery works. If you have a bad SEI, it doesn’t. The reason that the capacity of your cell phone battery slowly decreases year after year is because SEI is expanding and consuming your electrolyte in the liquid-based battery.
Andrew Westover, Study Co-Led Author, Oak Ridge National Laboratory
As far as a LiPON-based solid-state battery is concerned, however, a thin SEI layer develops to passivate lithium, thereby making it unreactive, and does not grow like the SEI in a traditional battery.
Researchers linked neutron reflectometry with electrochemistry to quantify this stable interphase between LiPON and lithium for the first time. It was as thin as 7 nm.
Westover stated, “We discovered with this study that the layer formed is about 70 atoms thick. This work shows it is possible to make interfaces in solid-state batteries that are thin and provide excellent performance.”
That small scale and the solid state of the materials drove the scientists to utilize neutrons to look into the battery’s interior.
Prior to the discovery of X-Rays, you couldn’t look under skin to see bones inside a body. You had to cut the skin open. Until now, that’s basically been the approach that most people have used to look at interphases in batteries. In this case, the scale is too small to cut anything open.
Andrew Westover, Study Co-Led Author, Oak Ridge National Laboratory
Westover added, “We needed a tool that would allow us to go through the material, to probe it non-destructively at that scale and understand what’s happening at the interphase. That’s where neutron reflectometry came in.”
Browning added, “We’re interested in how a battery is performing, so we need a way of looking inside while it’s doing its thing, operating on a length scale that’s important to the functioning of the device, to explore stability, long-term cyclability, etc.
Browning stated, “Because neutrons are weakly interacting, we can get them to the point we want to probe without any interference and then, more importantly, get them back out so we can determine what happened at the place of interest—the interphase in this case.”
Coupling neutron reflectometry with electrochemistry expedited the knowledge of the interphase between lithium metal and solid electrolytes present in solid-state batteries.
Westover stated, “This combination of techniques opens the door for us to look at the entire spectrum of solid-state electrolyte materials and determine which ones will enable your fast-charging, high-energy batteries. We’ve already started version 2.0, where we’re looking at a different type of solid electrolytes and starting to understand what they look like.”
He added, “New materials need to be invented that have this stability.” The design of future high-performance batteries will depend on it.
The study was financially supported by DOE’s Office of Energy Efficiency and Renewable Energy for the Vehicle Technologies Office. This study performed made use of resources at SNS, a DOE Office of Science user facility functioned by ORNL.
Journal Reference:
Browing, K. L., et al. (2023) In Situ Measurement of Buried Electrolyte–Electrode Interfaces for Solid State Batteries with Nanometer Level Precision. ACS Energy Letters. doi.org/10.1021/acsenergylett.3c00488.