It sounds magical: photoelectrodes that have the potential to transform the greenhouse gas CO2 back into N2 or methanol molecules into useful fertilizer by just using the energy of sunlight.
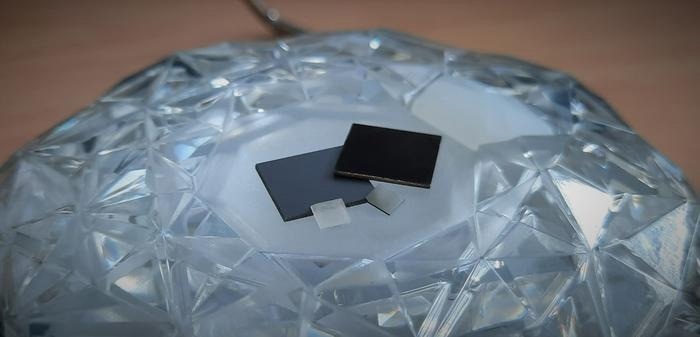
Four diamond materials are shown here: “Diamond black” made of polycrystalline nanostructured carbon (top right), the same material before nanostructuring (top left), an intrinsic single crystal (bottom left), and a single crystal doped with boron (bottom right). Image Credit: A. Chemin/ Helmholtz-Zentrum Berlin
A Helmholtz-Zentrum Berlin (HZB) study has shown that diamond materials are, in principle, ideal for such photoelectrodes.
By integrating X-Ray spectroscopic methods at BESSY II with other measurement techniques, Tristan Petit’s team has been successful in accurately monitoring which processes have been excited by light and also the vital role of the surface of the diamond materials.
While lab-grown diamond materials do not resemble the dazzling ones seen in jewelry stores, they have a lot to offer. These diamonds are frequently opaque and dark in appearance.
However, despite their unimpressive appearance, they hold great promise for a wide range of applications, for instance, in quantum sensors, computers, and brain implants, as well as metal-free photoelectrode in photo-electrochemical energy conversion.
They are completely viable and composed of carbon only, they degrade little in time in comparison to metal-based photoelectrodes, and they could be produced industrially.
Diamond materials are known to be ideal as metal-free photoelectrodes since, when excited by light, they can release electrons in water and activate chemical reactions that are hard to start otherwise.
A concrete example is the reduction of CO2 to methanol, which turns the greenhouse gas into a useful fuel. Also, it would be exciting to utilize diamond materials to transform N2 into nitrogen fertilizer NH3, utilizing much less energy compared to the Haber-Bosch process.
But diamond electrodes oxidize in water and oxidized surfaces, it was assumed, no longer release electrons into the water. Besides, the bandgap of diamond comes in the UV range (at 5.5 eV), so visible light is unlikely to be enough to excite the electrons.
Despite this expectation, earlier studies performed have shown mysterious emissions of electrons from visible light excitation. A new study by Dr. Tristan Petit’s group at HZB currently brings new knowledge and provides cause for belief.
Dr. Arsène Chemin, a postdoctoral researcher in Petit’s team, analyzed samples of diamond materials that have been produced at the Fraunhofer Institute for Applied Solid State Physics in Freiburg.
To streamline the CO2 reduction reaction, the samples were fabricated: doped with boron to ensure good electrical conductivity and nanostructured, providing huge surfaces to increase the emission of charge carriers like electrons.
Chemin utilized around four X-Ray spectroscopic methods at BESSY II to identify the surface of the sample and the energy that has been required to excite particular electronic surface states.
Further, Chemin utilized the surface photovoltage quantified in a specialized laboratory at HZB to identify which ones of these states are excited and how the charge carriers are moved in the samples.
Chemin quantified the photoemission of electrons of samples either in air or in liquid. By integrating these outcomes, Chemin could draw a comprehensive picture of the processes initially that occur on the surfaces of the sample following excitation by light.
Surprisingly, we found almost no difference in the photoemission of charges in liquid, regardless of whether the samples were oxidized or not.
Dr. Arsène Chemin, Postdoctoral Researcher, Helmholtz-Zentrum Berlin
This shows that diamond materials are ideally suited for use in aqueous solutions. Also, excitation with visible light is feasible: in the case of the boron-doped samples, violet light (3.5 eV) is enough to excite the electrons.
These results are a great cause for optimism. With diamond materials, we have a new class of materials that can be explored and widely used.
Dr. Arsène Chemin, Postdoctoral Researcher, Helmholtz-Zentrum Berlin
Furthermore, the methodology of this study is fascinating.The combination of such various spectroscopic methods could result in innovation in other photoactive semiconductor materials, stresses the physicist.
Journal Reference
Chemin, A., et al. (2023) Surface-Mediated Charge Transfer of Photogenerated Carriers in Diamond. Small Methods. doi.org/10.1002/smtd.202300423.