Ammonia (NH3), one of the most ubiquitous industrial chemicals, is required to manufacture nitrogenous fertilizer and can potentially be a next-generation green fuel. The reaction of fossil fuel-derived hydrogen and nitrogen (Haber-Bosch process) under high temperature (~500 °C) and high pressure (>15 MPa) uses 2% of the global power and emits ~1.5% of global greenhouse gases.
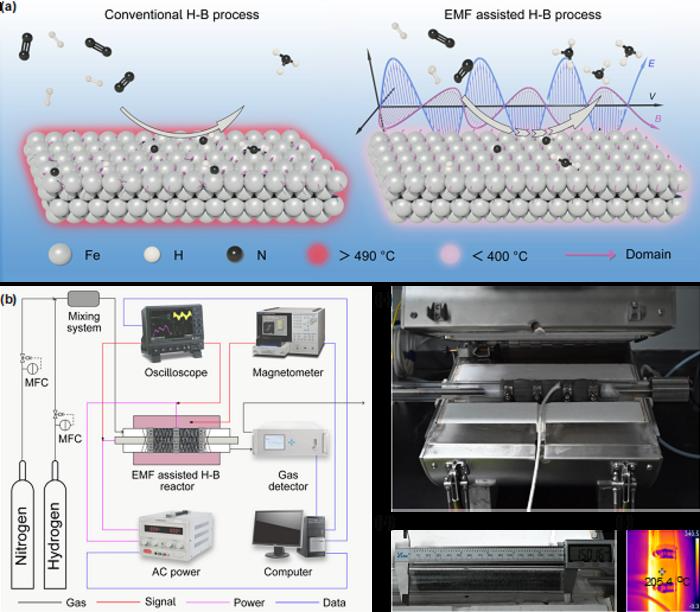 (a) Working principle for conventional and EMF-assisted H-B strategies. (b) The technological flow diagram of EMF-assisted ammonia synthesis. (c) Digital photograph of the EMF reactor. (d) Digital photograph of a quartz tube filled with 80 g Fe-based catalyst for the scale-up experiment. (e) Infrared thermal imaging of the reaction zone with external heating of 200 ℃. Image Credit: Science China Press
(a) Working principle for conventional and EMF-assisted H-B strategies. (b) The technological flow diagram of EMF-assisted ammonia synthesis. (c) Digital photograph of the EMF reactor. (d) Digital photograph of a quartz tube filled with 80 g Fe-based catalyst for the scale-up experiment. (e) Infrared thermal imaging of the reaction zone with external heating of 200 ℃. Image Credit: Science China Press
Using commercial iron-based catalysts, the Tianjin University team devised an electromagnetic field (EMF)-assisted H-B method for ammonia synthesis under moderate circumstances. With EMF aid, the onset temperature is 100 °C, which is obviously lower than the onset temperature without EMF support (300 °C).
The EMF-assisted H-B approach boosts ammonia yield (5 times) while decreasing energy usage (~2.7 times) in a typical scale-up experiment with 80 g commercial catalysts. The EMF induces higher electron transfer from Fe orbitals to NN orbitals in both side-on and end-on adsorption modes, which results in improved catalytic performance.
At Tianjin University, they built a pilot-scale system with a production capacity of 10,000 kg per year for EMF-assisted heat catalysis, moving the first step from the laboratory to the industrialization of EMF-assisted thermal catalysis.
They are conducting additional scale-up research on EMF-assisted thermal catalysis in Qinghai Province, which is rich in renewable energy, to investigate a viable industrial path for achieving large-scale storage and transportation bottlenecks of renewable energy to foster the early recognition of the “dual carbon” goal.
Journal Reference:
Zhang, B. S., et al. (2023) Electromagnetic field-assisted low-temperature ammonia synthesis. Science Bulletin. doi:10.1016/j.scib.2023.07.037