Global fossil fuel consumption has resulted in excessive CO2 emissions into the atmosphere, posing a major threat to the environment and creating an energy crisis. A viable method for converting CO2 into useful energy substances is electrochemical CO2 reduction (ECR), which uses renewable electric energy to reduce CO2 and produce usable energy fuels at the same time. Diverse ECR catalysts have been produced recently for the reduction of CO2, and remarkable efficiencies have also been attained.
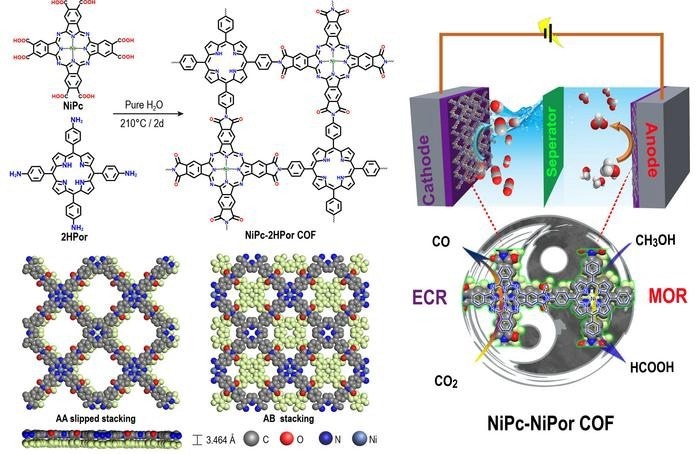 Schematic representation of polyimide linked metallophthalocyanine-porphyrin COFs for co-electrolysis of CO2 and methanol. Image Credit: Science China Press
Schematic representation of polyimide linked metallophthalocyanine-porphyrin COFs for co-electrolysis of CO2 and methanol. Image Credit: Science China Press
Nevertheless, a significant amount of energy was wasted because the majority of research only addressed the ECR half-reaction at the cathode and ignored the pertinent oxidation half reaction on the anode side. The majority of studies that have been published have combined the anode reaction treatment protocol with water oxidation processes (also known as oxygen evolution reactions, or OER).
Unfortunately, because of the H2O oxidation reaction’s slow kinetics and unfavorable thermodynamics, this OER process will result in a very large overpotential and significant energy input. Furthermore, when compared to many industrial chemicals, the created O2 has comparatively less value added. To replace the OER process, it is imperative to design oxidation reactions with great energy efficiency.
Due to the low theoretical overpotential, the application of the anodic oxidation process to organic molecules oxidative synthesis, such as methanol oxidation reaction (MOR) to generate HCOOH can successfully improve energy efficiency.
However, enabling these two electrocatalytic reactions to collaborate effectively remains a significant challenge. The key challenge in this field is a dearth of highly active electrocatalysts to carry out these two reactions.
The electrocatalysts for ECR and MOR should theoretically meet the following criteria: (1) highly active and accessible catalytic sites for reduction or oxidation reactions; (2) affinity and adsorption activation for substrates such as CO2 or methanol; (3) preferable electron and proton transfer ability; and (4) high stability during electrochemical measurements.
The development of bifunctional heterogeneous catalysts, which are employed for ECR and MOR simultaneously, can efficiently tackle the challenges above but has received little attention.
COFs, which have good structural designability, are potential substrates for catalytic processes. Some COF building blocks, in particular, have adequate coordination sites, allowing them to introduce metal active sites for conventional catalysis.
Recently, metallophthalocyanine (MPc) and metalloporphyrin (MPor) based-COFs for catalytic reactions have been investigated. Nonetheless, most studies primarily looked at the catalytic activity of a single functional component, leaving the integration of MPc and MPor into COFs for bifunctional catalysts untested.
Furthermore, Pc-based COFs, as one of the most important types of crystalline COFs, have high conductivity, mechanical performance, and redox-active characteristics. However, typical solvothermal production of Pc-based COFs requires the use of hazardous chemical solvents and catalysts. As a result, it is critical to create environmentally friendly and efficient ways of producing Pc-based COFs.
On the basis of the aforementioned research findings, Lan et al. rationally created NiPc-2HPor COF by hydrothermally condensing the phthalic acid group of NiPc and the aromatic amine group of 2HPor and then synthesized NiPc-NiPor COF via a post-synthesis coordination process.
Polyimide-linked COFs (PI-COFs) were synthesized and demonstrated strong chemical stability and activity for electrocatalysis MOR and ECR. Above all, the synthesized NiPc-MPor COFs combine crystalline and conductivity properties, as well as several active sites with diverse chemical environments for ECR and MOR.
Among these, the NiPc-NiPor COF demonstrates outstanding long-term stability and excellent activity for cathodic ECR and anodic MOR to HCOOH. The essential intermediates for both ECR and MOR were identified using in-situ fourier transform infrared spectroscopy (FT-IR).
Furthermore, density functional theory (DFT) simulations show that the ECR process primarily operates on NiPc units with the help of NiPor, whereas the MOR process prefers NiPor and conjugates with NiPc.
NiPc and NiPor’s synergistic catalytic effect adds to such high catalytic activity. This is the primary report of bifunctional MPc-MPor-based COFs for electrocatalytic cathodic ECR and anodic MOR at the same time, and it is also important in the field of bifunctional electrocatalysts.
Journal Reference:
Zhang, M., et al. (2023) Green synthesis of bifunctional phthalocyanine-porphyrin cofs in water for efficient electrocatalytic CO2 reduction coupled with methanol oxidation. National Science Review. doi:10.1093/nsr/nwad226