Hollow-structured reinforced metal catalysts such as nanoreactor catalysts with encapsulated active sites and well-defined shells offer an ideal place for multicomponents to respond or alter cooperatively, and it is distinguished as one of the famous catalyst candidates.
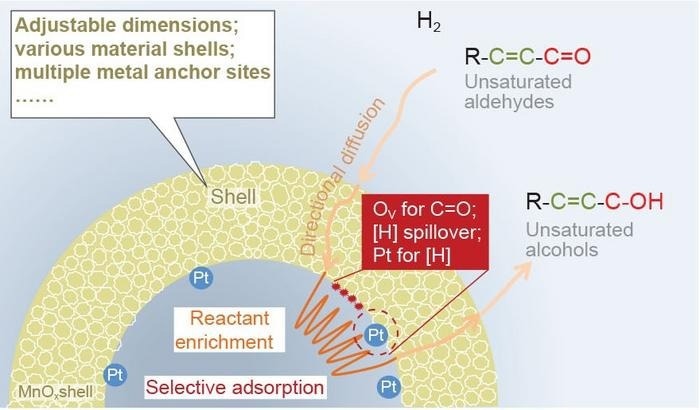 The picture shows the mechanism of a nanoreactor. The hollow structure of the pt nps@mnox nanoreactor affords a confined space in which reactants enter via directional diffusion driven by the concentration gradient. Then, selective adsorption reduces the internal reactant concentrations, which would promote the in-diffusion of reactants consistently. After the reaction with abundant hydrogen atoms, the weak adsorption of products forces them to leave the nanoreactor in time. Image credit: Science China Press
The picture shows the mechanism of a nanoreactor. The hollow structure of the pt nps@mnox nanoreactor affords a confined space in which reactants enter via directional diffusion driven by the concentration gradient. Then, selective adsorption reduces the internal reactant concentrations, which would promote the in-diffusion of reactants consistently. After the reaction with abundant hydrogen atoms, the weak adsorption of products forces them to leave the nanoreactor in time. Image credit: Science China Press
The study of the enhancement effect at the mesoscale (500-2000 nm) is not inclusive. However, reactant enhancement has been anticipated by examining the relationship between the catalytic performance and the structure of nanoreactors at the nano level.
Building the nanoreactor models with active metals inside and outside the hollow nanostructure via different synthetic methods or sequences certainly influences the microenvironment around the active sites, as well as the essential active sites.
Moreover, enhancing reactant concentration at the mesoscale entails many processes, including adsorption and diffusion, which are beyond the scope of detailed description through the creation of simplistic computational models at the nanoscale level. Examination of the reactant enrichment at the mesoscale level needs to sustain the intrinsic active sites constant while building the research model with or without enrichment behavior.
Researchers at the Dalian Institute of Chemical Physics (DICP), University of Chinese Academy of Sciences, Taiyuan University of Technology, University of Surrey, and Inner Mongolia University prompt a new nanoreactor catalyst (Pt NPs@MnOx) with uniformly dispersed Pt nanoparticles. The particles are encapsulated in an oxygen vacancy-rich MnOx hollow structure to catalyze the selective hydrogenation of CAL and inspect reactant enrichment at the mesoscale level.
The study was published in the Beijing-based National Science Review
The catalytic performance for CAL-selective hydrogenation on Pt NPs@MnOx is 3.4-fold higher than that of Pt NPs and MnOx, which is substantially crushed into an open structure. UV–vis, in situ FTIR, and IGA measurements validate that hollow MnOx shell of Pt NPs@MnOx leads to higher CAL uptake.
The mechanism underlying this phenomenon may involve a two-step process. In the first step, the hollow structure establishes a confined space, facilitating the directional diffusion of outer reactants into the interior of the hollow structure. This diffusion is driven by the concentration gradient and/or a capillary-like effect.
Subsequently, these reactants become immobilized on the inner surface through adsorption, ensuring a localized low concentration within the confined space. In contrast, Pt NPs&MnOx are unable to facilitate this directional diffusion process. Density Functional Theory (DFT) results also indicate that CAL exhibits stronger adsorption on the surface of Pt NPs@MnOx compared to Pt NPs and MnOx when excess reactants are present, marking the second step in this process.
H2-TPR–MS and Finite-element simulation results also revealed that Pt NPs@MnOx nanoreactor generates a stable space with higher concentration and less flow rate to avoid the escape of the reactants such as dissociated hydrogen.
Hence, it is evident that the enrichment of reactants results from the directional diffusion of reactants driven by a local concentration gradient and the increased amount of reactant adsorption, primarily facilitated by the enhanced adsorption capability within the hollow MnOx structure.
Pt NPs@MnOx catalyst exhibits extremely high catalytic activities and selectivity in various reaction pressures. A 95% conversion with 95% COL selectivity is obtained on Pt NPs@MnOx at only 0.5 MPa H2 and 40 min, a comparatively mild condition compared with many described catalytic systems.
By amalgamating experimental findings with Density Functional Theory calculations, it becomes evident that the superior selectivity for cinnamyl alcohol (COL) arises from the selective adsorption of cinnamaldehyde (CAL) and the rapid formation and desorption of COL within the MnOx shell.
Furthermore, the presence of a hollow void within the structure induces the behavior of reactant enrichment, which in turn enhances the catalytic activity. These discoveries open up the possibility of enhancing catalytic performance at the mesoscale level by purposefully designing a rational nanoreactor, as opposed to merely diminishing the size of metal particles or modifying them with nanoscale-level heteroatoms or ligands.
Journal Reference:
Xu, W., et al. (2023). SLIPS-TENG: robust triboelectric nanogenerator with optical and charge transparency using a slippery interface. National Science Review. doi.org/10.1093/nsr/nwz025