Researchers from the Institute for Basic Science (IBS) Center for Nanoparticle Research, under the direction of Professor Kisuk Kang, have made significant progress toward developing next-generation solid-state batteries. Their new discoveries are expected to pave the way for the development of batteries built on a unique solid electrolyte based on chloride that has remarkable ionic conductivity.
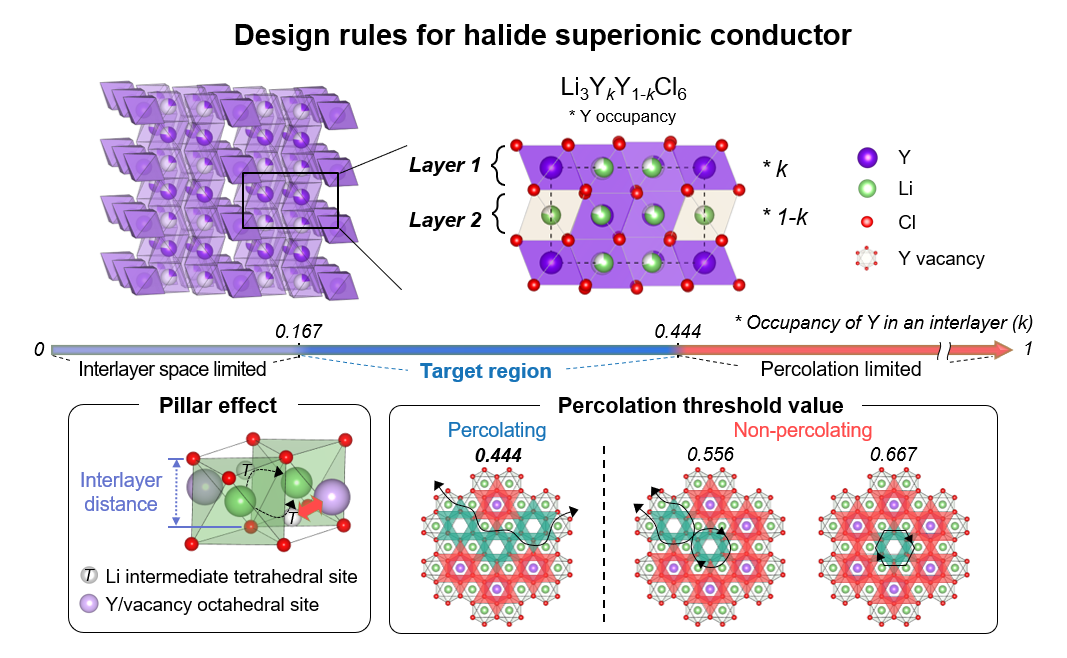
Design Strategy for Trigonal Chloride Solid Electrolytes. The arrangement of metal ions (yttrium in this case) within each layer affects the ionic conductivity. To ensure the unobstructed movement of lithium ions, the number of metal ions occupying available sites within each layer should be less than 0.444. Furthermore, to create a sufficiently wide pathway for lithium ions within each layer, the occupancy of metal ions should be more than 0.167. Therefore, achieving an occupancy of metal ions between 0.167 and 0.444 within each layer results in a conductive layer with high ionic conductivity. Image Credit: Institute for Basic Science
A major issue with today’s commercial batteries is their dependency on liquid electrolytes, which increases the risk of flammability and explosion. As a result, the development of non-combustible solid electrolytes is critical for the advancement of solid-state battery technology.
In the ongoing worldwide transition toward sustainable transportation, as the world prepares to regulate internal combustion engine vehicles and expand the usage of electric vehicles, research into the key components of secondary batteries, particularly solid-state batteries, has gained significant momentum.
To make solid-state batteries practicable for everyday use, materials with high ionic conductivity, strong chemical and electrochemical stability, and mechanical flexibility must be developed. While previous research yielded sulfide and oxide-based solid electrolytes with excellent ionic conductivity, none of these materials matched all of these requirements.
Scientists have previously investigated chloride-based solid electrolytes, which are known for their superior ionic conductivity, mechanical flexibility, and high-voltage stability. Due to these characteristics, some believe chloride-based batteries are the most plausible candidates for solid-state batteries.
However, these hopes quickly died out, as the chloride batteries were regarded as impractical due to their heavy reliance on expensive rare earth metals, such as yttrium, scandium, and lanthanide elements, as secondary components.
To tackle these issues, the IBS research team examined the dispersal of metal ions within chloride electrolytes. They believed the reason trigonal chloride electrolytes can achieve low ionic conductivity is based on the variation of metal ion arrangements within the structure.
They first tested this theory on lithium yttrium chloride, a common lithium metal chloride compound. The journey of the lithium ions was impeded by electrostatic forces when the metal ions were in close proximity to their path. On the other hand, the mobility of lithium ions was hindered if the metal ion occupancy was too low because the channel became too narrow.
The study team successfully developed a solid electrolyte with high ionic conductivity by introducing ways to design electrolytes in a way that mitigates these competing aspects, building on these discoveries.
The team went one step further to effectively illustrate this approach by developing a zirconium-based solid-state battery that contains lithium metal chloride, which is significantly less expensive than versions that use rare earth metals. This was the first time that the impact of the arrangement of metal ions on the ionic conductivity of a material was shown.
This study highlights the significance of metal ion distribution, which is frequently disregarded, in the ionic conductivity of solid electrolytes based on chlorides. The IBS Center’s research is anticipated to further propel the commercialization of solid-state batteries and open the door for the development of new chloride-based solid electrolytes, offering enhanced energy storage cost and safety.
This newly discovered chloride-based solid electrolyte is poised to transcend the limitations of conventional sulfide and oxide-based solid electrolytes, bringing us one step closer to the widespread adoption of solid-state batteries.
Kisuk Kang, Study Corresponding Author and Professor, Center for Nanoparticle Research, Institute for Basic Science
This study was published on November 3, 2023, in Science, one of the world's most prestigious scientific journals.
Journal Reference:
Yu, S., et al. (2023) Design of a trigonal halide superionic conductor by regulating cation order-disorder. Science. doi:10.1126/science.adg6591