The working principle of so-called promoters in a catalytic process has been effectively seen in real-time for the first time by researchers at TU Wien. Although their significance in technology is significant, little is known about how these promoters operate.
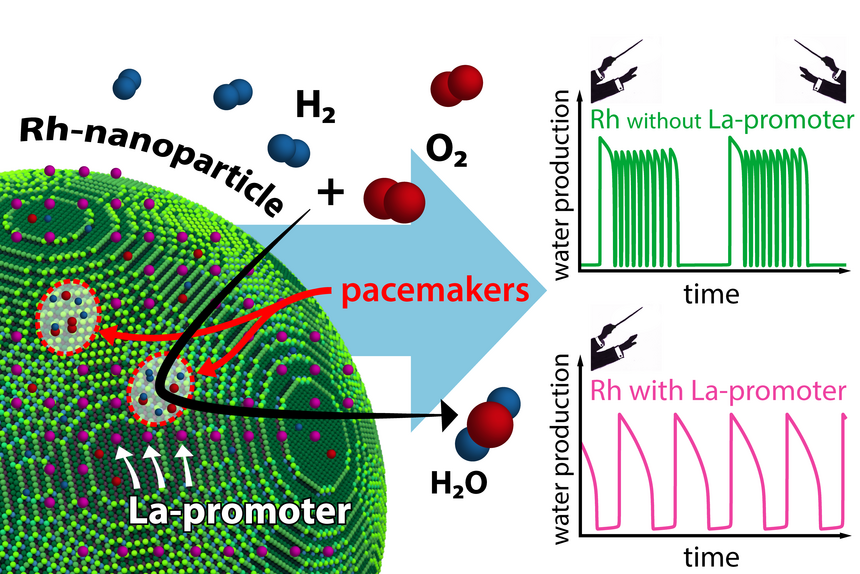 The reaction behavior of an individual nanoparticle is determined by its pacemakers. Addition of a La promoter significantly influences the interaction of these pacemakers. Image Credit: TU Wien.
The reaction behavior of an individual nanoparticle is determined by its pacemakers. Addition of a La promoter significantly influences the interaction of these pacemakers. Image Credit: TU Wien.
Chemical processes involving the generation of valuable chemicals and energy carriers as well as the cleaning of exhaust gases all depend on catalysts. Catalysts are often enhanced by the addition of minute amounts of other chemicals. Such substances are called “promoters.” Despite being extremely important to technology, they have always been challenging to study.
Most of the time, identifying which quantity of promoters has what effects on a catalyst has been a trial-and-error procedure. However, TU Wien researchers were able to directly observe the action of lanthanum promoters in hydrogen oxidation. They visualized the role of individual La atoms using high-tech microscopy tools.
Their research discovered that two surface portions of the catalyst function as pacemakers, much like conductors in an orchestra. The promoter is crucial in their relationship since he controls the pacemakers. This study’s findings have recently been published in the journal Nature Communications.
Watching the Reaction Live
Many chemical processes use catalysts in the form of tiny nanoparticles.
Günther Rupprechter, Professor, Institute of Materials Chemistry, TU Wien
While the performance of catalysts can be easily verified through product analysis, microscopic insights cannot be obtained using this method.
This is no longer the case. Günther Rupprechter and his colleagues have spent years developing advanced technologies for directly monitoring individual nanoparticles during a chemical reaction. This enables examining how activity changes at different locations on these nanoparticles during the process.
Rupprechter added, “We use rhodium nanotips that behave like nanoparticles. They can serve as catalysts, for example, when hydrogen and oxygen are combined to form water molecules – the reaction we are examining in detail.”
Oscillating Between “Active” and “Inactive”
The TU Wien team has already shown in recent years that distinct areas of nanoparticle surfaces display distinct behaviors, oscillating between an active and an inactive state. There are times when the right chemical reaction happens where it should and times when it does not.
It has been demonstrated with specialized microscopes that several types of these oscillations happen on every nanoparticle simultaneously and that they all affect one another. Often just a few atom diameters wide, some areas of the nanoparticle surface are more important than others. They function as extremely effective “pacemakers,” even regulating the chemical oscillations in other areas.
Promoters can now intervene in this pacemaker operation, which is exactly what researchers at TU Wien have been able to examine. Lanthanum can operate as a catalyst promoter when rhodium is utilized as a catalyst.
Individual lanthanum atoms were inserted on a rhodium nanoparticle’s small surface. The same particle was studied both with and without the promoter. This method revealed the precise effect of individual lanthanum atoms on the progression of the chemical process.
Lanthanum Changes Everything
The experiments were carried out by Maximilian Raab, Johannes Zeininger, and Carla Weigl.
The difference is enormous. A lanthanum atom can bind oxygen, and that changes the dynamics of the catalytic reaction.
Maximilian Raab, Research Assistant, TU Wien
Lanthanum, in trace amounts, affects the connection between distinct sections of the nanoparticle.
Lanthanum can selectively deactivate certain pacemakers. Imagine an orchestra with two conductors – we would hear quite complex music. The promoter ensures that there is only one pacemaker left, making the situation simpler and more ordered.
Johannes Zeininger, Univ.-Assistant Postdoc, TU Wien
In addition to the observations, the team, led by Alexander Genest and Yuri Suchorski, created a mathematical model to mimic the interaction between the various sections of the nanoparticle.
This technique provides a more powerful way to characterize chemical catalysis than previous approaches by considering how distinct parts of the catalyst swing between activity and inactivity and, governed by promoters, mutually impact one other.
The Austrian Science Fund (FWF; P32772-N and SFB TACO F81-P08) funded the research.
Journal Reference:
Raab, M., et al. (2023) Lanthanum modulated reaction pacemakers on a single catalytic nanoparticle. Nature Communications. doi:10.1038/s41467-023-43026-3.