Reviewed by Lexie CornerMar 19 2024
In partnership with POSTECH and Yonsei University, Dr. Hyung-Suk Oh and Dr. Woong-Hee Lee of the Clean Energy Research Center at the Korea Institute of Science and Technology (KIST) developed a methodology using bifunctional platinum-nickel alloy catalysts with an octahedral structure that exhibits both oxygen reduction and generation reactions, to improve the reversibility and durability of electrodes.
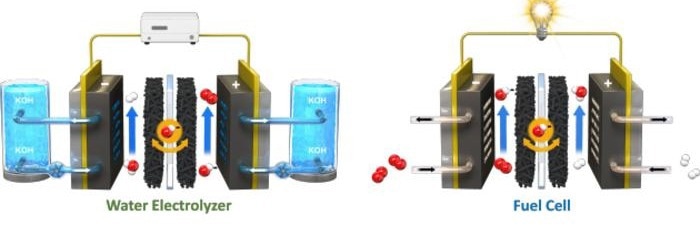 Unitized Renewable Fuel Cells operation schematic. Image Credit: Korea Institute of Science and Technology
Unitized Renewable Fuel Cells operation schematic. Image Credit: Korea Institute of Science and Technology
A novel class of bifunctional catalysts uses a single catalyst to create oxygen and hydrogen from water simultaneously. Currently, separate catalysts are used for both electrodes in electrochemical systems like water electrolysis technology and CCU (Carbon dioxide Capture and Utilization), raising the cost of hydrogen per unit.
Conversely, bifunctional catalysts that can be produced in a single step are becoming increasingly popular as a technique, helping to lower manufacturing costs and boost the profitability of electrochemical energy conversion systems.
The issue with bifunctional catalysts is that following each electrochemical reaction that produces oxygen and hydrogen, the electrode material undergoes structural changes that impair the efficiency of subsequent reactions. Therefore, it is critical to ensure reversibility and durability to sustain the catalyst structure long after the reaction to commercialize bifunctional catalysts.
The scientists used platinum and nickel, which perform well in oxygen reduction and generation reactions, to create alloy catalysts with various structures that improved the bifunctional catalyst's reversibility and durability.
According to the experimental findings, the octahedral structure exhibited the highest level of nickel-platinum interaction. In oxygen reduction and generation reactions, the alloy catalysts outperformed the platinum and nickel monoliths by a factor of more than two.
The source of the performance loss was found to be the production of platinum oxide during the alloy catalyst's repeated generation reaction. The researchers then created a structure restoration technology to convert platinum oxide to platinum.
The catalyst's shape was successfully restored in large-area reactor studies for commercialization, and the scientists also more than doubled the catalyst's run time. This was verified by transmission electron microscopy.
By substituting bifunctional catalysts for separate catalysts for oxygen evolution and reduction processes, the team's structure recovery methods and bifunctional catalysts are intended to expedite the commercialization of unitized renewable fuel cells (URFCs) technology. Dual-purpose URFCs that generate electricity and hydrogen can reduce production costs by minimizing the need for costly catalysts while preserving output.
The technology to improve the reversibility and durability of catalysts has provided a new direction for the development of bifunctional catalysts, which is an important technology for electrochemical energy conversion systems, and it will contribute to the commercialization and carbon neutrality of electrochemical systems such as URFCs in the future.
Hyung-suk Oh, Study Lead Researcher, Korea Institute of Science and Technology
Journal Reference:
Oh, C., et al. (2023) Activity Restoration of Pt–Ni Octahedron via Phase Recovery for Anion Exchange Membrane‐Unitized Regenerative Fuel Cells. Advanced Energy Materials. doi.org/10.1002/aenm.202302971