Water electrolysis, a system for generating hydrogen through water electrolysis, is an eco-friendly technology for producing hydrogen fuel, a promising future energy source devoid of environmental pollutants. However, its drawbacks include low hydrogen production efficiency and high production costs.
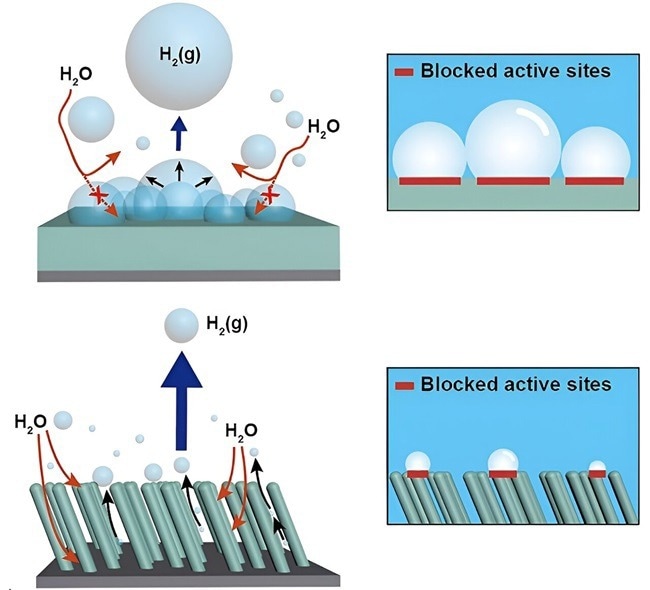 The team created a porous nickel catalyst material featuring a nanorod protrusion structure, incorporating efficient three-dimensional pore channels and superaerophobic surface wettability. This design aims to facilitate the rapid separation of hydrogen bubbles from the catalyst surface. The outcome is a significant enhancement in the efficiency of hydrogen production within the water electrolysis system when compared to traditional bulk thin film-shaped catalyst electrodes. Image Credit: Pohang University of Science and Technology
The team created a porous nickel catalyst material featuring a nanorod protrusion structure, incorporating efficient three-dimensional pore channels and superaerophobic surface wettability. This design aims to facilitate the rapid separation of hydrogen bubbles from the catalyst surface. The outcome is a significant enhancement in the efficiency of hydrogen production within the water electrolysis system when compared to traditional bulk thin film-shaped catalyst electrodes. Image Credit: Pohang University of Science and Technology
Researchers from Pohang University of Science and Technology (POSTECH) have simultaneously overcome both issues. The research was published in the journal Advanced Materials.
A collaborative research team led by Professor Jong Kyu Kim, Jaerim Kim, a Ph.D. Candidate, Professor Yong-Tae Kim, and Doctor Sang-Mun Jung from the Department of Materials Science and Engineering at POSTECH have succeeded in developing an affordable and effective water electrolysis catalyst using an oblique angle deposition method and nickel (Ni), that overcomes the limitations of conventional catalysts.
Water electrolysis techniques are expensive because they use precious metals like platinum as catalysts to produce hydrogen. Moreover, using traditional thin-film catalysts frequently leads to insufficient hydrogen bubble separation, which obstructs the catalyst’s active sites or restricts the flow of reactants, both of which reduce process efficiency.
The study team decided to use nickel and oblique angle deposition to solve these problems. This method, which provides a simple and affordable solution, entails tilting the substrate during deposition to readily form various nanostructures of the material. Furthermore, nickel is a non-precious metal catalyst that is widely available on Earth and has a comparatively high efficiency in the production of hydrogen.
The researchers synthesized nickel with beautifully sculpted, vertically aligned nanorod protrusions using an oblique angle deposition approach. To address the hydrogen adhesion problems, the researchers built a highly porous nickel nanorod array, which provides a unique superaerophobic surface feature. This is in contrast to ordinary nanostructures, which only increase the catalyst’s surface area.
According to experimental data, hydrogen bubbles produced during electrolysis separated from the superaerophobic surface more quickly. With efficient pore channels, the team’s three-dimensional, superaerophobic nickel nanorod catalyst showed an astounding 55-fold increase in hydrogen production efficiency over the same quantity of nickel in a conventional thin film structure.
Professor Jong Kyu Kim and Ph.D. Jaerim Kim, leading the research, said, “By enhancing the efficiency of the water electrolysis process for green hydrogen production, we are advancing towards a hydrogen economy and a carbon-neutral society. This breakthrough not only benefits water electrolysis but also holds promise for various other renewable energy applications where surface reactions play a crucial role, such as carbon dioxide reduction and light energy conversion systems.”
Journal Reference:
Kim, J., et al. (2023) Efficient Alkaline Hydrogen Evolution Reaction Using Superaerophobic Ni Nanoarrays with Accelerated H2 Bubble Release. Advanced Materials. doi.org/10.1002/adma.202305844.