Reviewed by Lexie CornerMar 25 2024
A group of scientists has discovered a viable and more environmentally friendly substitute for the traditional method of producing ammonia.
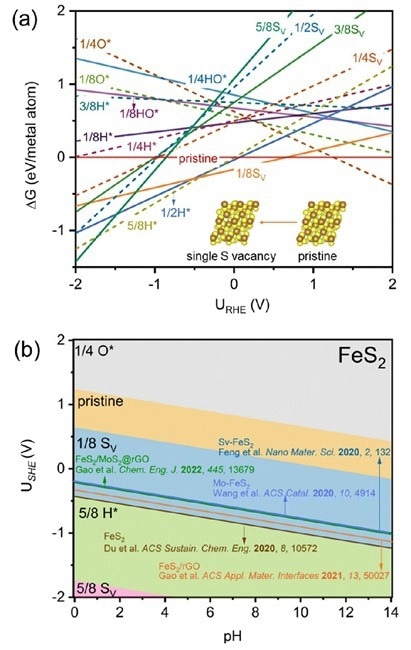 Alculated (a) 1D and (b) 2D surface Pourbaix diagrams of FeS2(111) considering different coverages of SV, O*, H*, and HO*. The experimental potentials at the highest faradaic efficiencies of reported FeS2-based catalysts are plotted for a direct comparison. Image Credit: Hao Li et al.
Alculated (a) 1D and (b) 2D surface Pourbaix diagrams of FeS2(111) considering different coverages of SV, O*, H*, and HO*. The experimental potentials at the highest faradaic efficiencies of reported FeS2-based catalysts are plotted for a direct comparison. Image Credit: Hao Li et al.
On February 21st, 2024, their study findings were published in the Journal of Materials Chemistry A.
In the early twentieth century, Fritz Haber and Carl Bosch found a method of synthesizing ammonia from nitrogen and hydrogen gas, allowing the chemical to be produced on an industrial scale. To this day, the Haber-Bosch synthesis is the primary method of manufacturing ammonia.
However, the approach has certain environmental downsides. It is energy and resource-expensive since manufacturing hydrogen gas frequently requires natural gas, which emits carbon dioxide as a byproduct.
The electrochemical nitrogen reduction reaction (ENRR), which converts nitrogen gas from the air into ammonia using an electrical current, is seen as a potential and sustainable alternative. However, developing high-performance and cost-effective ENRR catalysts remains a significant problem for commercial-scale ambient ammonia production.
We explored the potential of less-precious transition metal disulfides (TMS2) as catalysts for ENRR. Through meticulous analysis of electrochemistry-induced surface states, we uncovered a previously unrecognized factor contributing to their high ENRR performance: S-vacancy generation.
Hao Li, Study Corresponding Author and Associate Professor, Advanced Institute for Materials Research, Tohoku University
Li and his colleagues began with a common ENRR TMS2 catalyst, iron disulfide (FeS2), and discovered that under ENRR circumstances, S-vacancies can easily form on the catalyst surface. Using advanced computational models, they proved that the electrochemistry-driven “in situ” production of S-vacancies significantly improves ENRR activity by enabling higher N-N adsorption and activation.
Experimental observations verified their findings, which were also compatible with previous research on ENRR potential windows that achieved optimum Faradaic efficiency—a measure of an electrochemical process’s effectiveness in turning electrical energy into chemical energy or vice versa.
Their study also included different TMS2 catalysts (SnS2, MoS2, NiS2, and VS2), indicating a universal phenomenon of “in situ” S-vacancy formation under ENRR potentials.
Li added, “Our research underscores the critical importance of considering surface states in the design of ENRR catalysts. By shedding light on the role of S-vacancies, we have provided a valuable roadmap for enhancing ENRR performance and accelerating the transition towards sustainable ammonia production.”
AIMR Fusion Research provided funding for this project, while the JSPS Postdoctoral Fellowship for Dr Tianyi Wang in the Hao Li Lab also received significant funding.
Journal Reference:
Wang, T., et. al. (2024) Origin of electrocatalytic nitrogen reduction activity over transition metal disulfides: critical role of in situ generation of S vacancy. Journal of Materials Chemistry A. doi:10.1039/D4TA00307A.