Zinc-nitrate batteries are a type of non-rechargeable energy storage technology that uses the redox potential difference between zinc and nitrate ions to store and release electrical energy. A research team led by chemists from the City University of Hong Kong (CityU) created a high-performance rechargeable zinc-nitrate/ethanol battery using a novel catalyst.
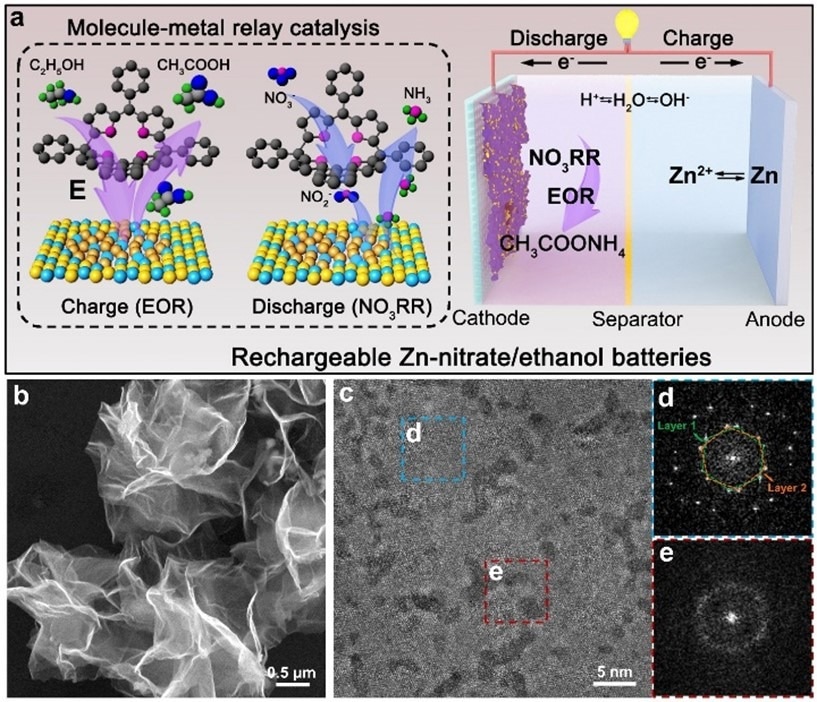 (a) Schematic illustration for the working principle of assembled Zn-nitrate/ethanol batteries. (b) SEM and (c) Low-dose HAADF-STEM images of as-synthesized RhCu M-tpp. (d, e) FFT patterns corresponding to the regions marked by the (d) blue and (e) red dash squares in (c). Image Credit: Zhou J., et al
(a) Schematic illustration for the working principle of assembled Zn-nitrate/ethanol batteries. (b) SEM and (c) Low-dose HAADF-STEM images of as-synthesized RhCu M-tpp. (d, e) FFT patterns corresponding to the regions marked by the (d) blue and (e) red dash squares in (c). Image Credit: Zhou J., et al
The team developed and produced an effective tetraphenylporphyrin (tpp) modified heterophase rhodium-copper alloy metallene (RhCu M-tpp). This bifunctional catalyst demonstrates remarkable capabilities in both the electrocatalytic nitrate reduction reaction (NO3RR) and the ethanol oxidation reaction (EOR) in a neutral medium, overcoming the monofunctional limitations of traditional metal-based solid catalysts and serving as an invaluable framework for future sustainable energy storage design.
This study highlights the significance of molecule-metal relay catalysis to efficient NH3 electrosynthesis in NO3RR and offers a multifunctional battery prototype that shows the benefits of metal-based hybrid electrochemical systems on high-performance, sustainable energy storage and conversion.
Fan Zhanxi, Study Lead and Assistant Professor, Department of Chemistry, City University of Hong Kong
Prof. Fan elaborated on the significance of the results, saying that the as-obtained RhCu M-tpp solves the problem that conventional Cu-based catalysts have in that they need a significant negative potential to effectively convert nitrate to ammonia while conducting NO3RR in a neutral medium.
Furthermore, to address the low rechargeability of conventional zinc-nitrate galvanic cells, a rechargeable Zn-nitrate/ethanol battery was successfully built based on the excellent bifunctionality of as-prepared RhCu M-tpp for both NO3RR and EOR.
The study also elucidated the process of molecule-metal relay catalysis, which involves the reduction of nitrate to nitrite on tpp followed by converting nitrite to ammonia on metallic sites. This confirmed that it is feasible to modify the molecular surface of nanometals to enhance their electrochemical performance for NO3RR.
Zinc-nitrate batteries depend on the activity of their cathode catalysts to operate. Nevertheless, there are currently drawbacks to catalysts based on copper. Their poor proton adsorption and high applied potential requirement lead to low ammonia production and current density. These catalysts are also unsuitable for the electrocatalytic oxygen evolution reaction (OER), which results in batteries that cannot be recharged and have a poor cycling life.
The study team created ultrathin bimetallic RhCu metallenes to lower the energy barrier for copper and solve these problems. After several efforts, they found that adding small molecules known as tpp to the RhCu metallenes’ surface greatly increased the efficiency of converting nitrate to ammonia without lowering the effectiveness of metallic substrates in ethanol oxidation. Thus, this discovery can potentially improve zinc-nitrate batteries’ overall performance.
The study’s conclusions present a practical method for building high-efficiency hybrid energy systems based on zinc and offer insightful information for developing future catalysts for multipurpose, eco-friendly devices.
Journal Reference:
Zhou, J., et. al. (2024) Constructing molecule-metal relay catalysis over heterophase metallene for high-performance rechargeable zinc-nitrate/ethanol batteries. PNAS. doi:10.1073/pnas.2311149120