Reviewed by Lexie CornerApr 10 2024
Researchers at the University of Michigan and Samsung's Advanced Materials Lab have developed a novel method for creating chemically complex materials. This method could make it easier to develop new chemistry for batteries, semiconductors, and other devices.
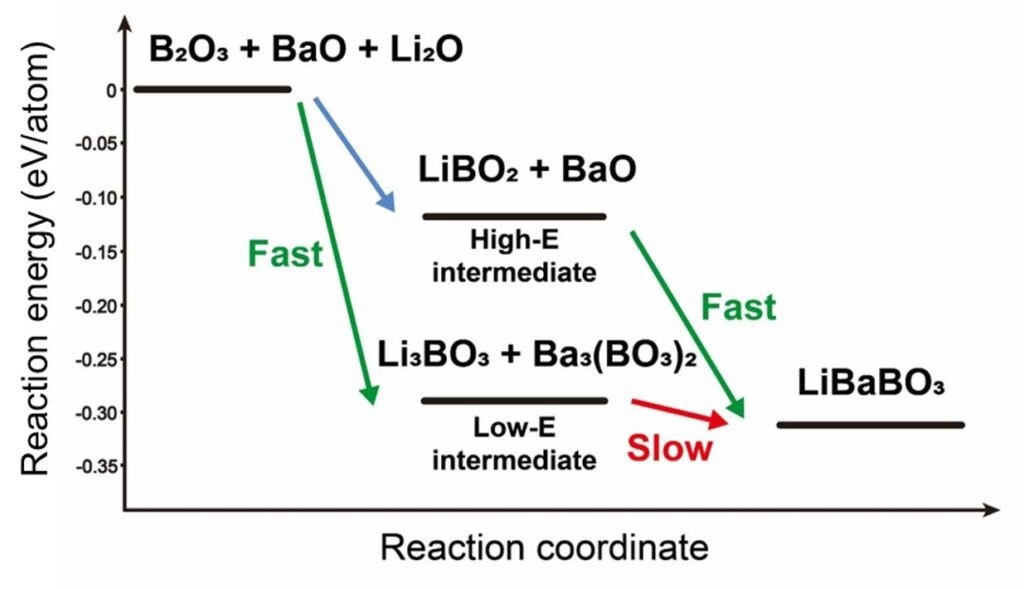 This reaction diagram shows how the order in which battery ingredients are mixed could impact the overall efficiency and purity of the desired material. To make lithium barium borate (LiBaBO3), chemists have to mix boron trioxide (B2O3), barium oxide (BaO), and lithium oxide (Li2O) and heat them in a furnace. When combining these ingredients, an intermediate compound can temporarily form before the desired material. As low-energy compounds are very stable, they are slow to react to form the final desired product, meaning they stick around as impurities in the final yield. However, if boron trioxide and lithium oxide are mixed separately first (the higher path in the chart), then the intermediates are less stable, so they will quickly form the final product with fewer impurities. Image Credit: Jiadong Chen, Wenhao Sun Research Group, University of Michigan
This reaction diagram shows how the order in which battery ingredients are mixed could impact the overall efficiency and purity of the desired material. To make lithium barium borate (LiBaBO3), chemists have to mix boron trioxide (B2O3), barium oxide (BaO), and lithium oxide (Li2O) and heat them in a furnace. When combining these ingredients, an intermediate compound can temporarily form before the desired material. As low-energy compounds are very stable, they are slow to react to form the final desired product, meaning they stick around as impurities in the final yield. However, if boron trioxide and lithium oxide are mixed separately first (the higher path in the chart), then the intermediates are less stable, so they will quickly form the final product with fewer impurities. Image Credit: Jiadong Chen, Wenhao Sun Research Group, University of Michigan
Their novel recipes combine unorthodox elements to produce battery materials with fewer impurities, resulting in less expensive refining processes and increased economic viability.
Over the past two decades, many battery materials with enhanced capacity, charging speed, and stability have been designed computationally but have not made it to market.
Wenhao Sun, Study Corresponding Author and Dow Early Career Professor, University of Michigan
Sun added, “A lot of times, a simple material is a good starting point, but when you add a little bit of compound A and a little bit of compound B, magic happens, and you get big improvements in capacity or charging rate. However, these chemically complex materials are often difficult to manufacture at scale with high purity.”
Usually, powders of several oxides are combined and fired to create battery materials. Instead of reacting simultaneously, these powders respond in a specific order. The ingredients that release the most energy during their reaction are usually the first two to react. When no more reactions are possible, the first reaction produces an intermediate compound that subsequently reacts with the remaining powder.
If the chemical bonds in the intermediate compounds are hard to break, they might not react completely with the other ingredients. When they do not fully react, the intermediates remain as undesirable contaminants in the final substance.
We designed a strategy to make impurity-free materials more reliably. The trick is to only work with two ingredients at a time, and deliberately make unstable intermediates that will react completely with the remaining ingredients.
Jiadong Chen, Study First Author and Doctoral Student, University of Michigan
To put this technique to the test, Sun’s team devised 224 distinct recipes for producing 35 different known materials comprising elements included in today’s batteries and next-generation ‘beyond-lithium’ batteries.
The researchers then collaborated with Samsung Semiconductor’s Advanced Materials Lab in Cambridge, Massachusetts, to determine if their recipes generated these 35 materials with fewer impurities than standard recipes. Samsung’s automated robotic lab can produce up to 24 distinct battery materials every 72 hours.
Robotic arms handle the ingredients and control the lab equipment that determines the purity of the resulting materials. Meanwhile, computers automatically record the outcomes of each experiment, generating a database that researchers can utilize to identify which recipes are most effective.
Chen added, “With the automatic lab, we could broadly test our hypothesis on diverse battery chemistries.”
The experiments confirmed that novel recipes, including unstable components, produced cleaner products. The new recipes increased the purity of the materials by up to 80 %, and six of the target materials could only be produced using the new recipes.
The team’s study included blueprints for the robotic lab, which Sun believes will encourage additional chemistry laboratories to use robotic labs for science and materials manufacturing.
“We need more data—not just from successful recipes but also the unsuccessful ones—to improve materials manufacturing strategies. More robotic labs will help generate the needed data,” Sun stated.
According to the researchers, these facilities are accessible to most research institutes and might considerably accelerate materials development.
The startup cost for the robotic equipment is about $120,000—not as high as you might think. But the payoffs in throughput, reliability, and data management are invaluable.
Yan Eric Wang, Study Co-Author, Principal Engineer and Project Manager, Advanced Materials Lab, Samsung
The US Department of Energy’s Basic Energy Sciences program supported the study.
Journal Reference:
Chen, J., et. al. (2024) Navigating phase diagram complexity to guide robotic inorganic materials synthesis. Nature Synthesis. doi:10.1038/s44160-024-00502-y