Researchers from Japan’s RIKEN Center for Sustainable Resource Science (CSRS), led by Satoshi Kamiguchi, have identified a more environmentally friendly technique to generate ammonia, a key component used in fertilizers. The study, published in the scientific journal Chemical Science, proposes a novel catalyst that works reliably at low temperatures, lowering the energy and money required to produce ammonia.
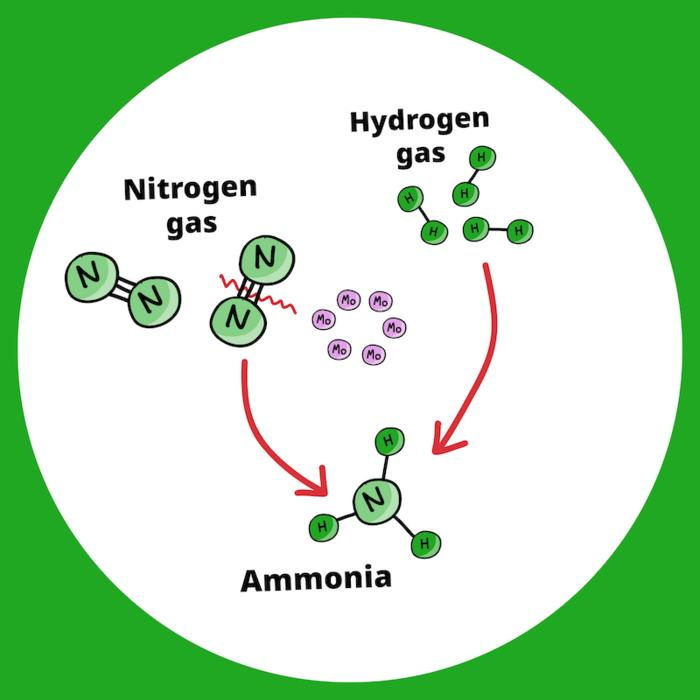 In this study, ultrasmall molybdenum (Mo) metal particles were used to help break the triple bonds between nitrogen atoms in nitrogen gas (N2) at lower than standard temperatures and pressure, thus allowing "greener" ammonia production that requires les energy. Image Credit: Song et al., doi 10.1117/1.JBO.29.S2.S22703
In this study, ultrasmall molybdenum (Mo) metal particles were used to help break the triple bonds between nitrogen atoms in nitrogen gas (N2) at lower than standard temperatures and pressure, thus allowing "greener" ammonia production that requires les energy. Image Credit: Song et al., doi 10.1117/1.JBO.29.S2.S22703
This finding will facilitate the transition from fossil fuels to a carbon-neutral and green energy economy since ammonia is an excellent substitute for fuel that can be used to securely store hydrogen.
Fertilizers deliver more nitrogen to plants, allowing them to thrive and boosting agricultural yields. The nitrogen in fertilizers is derived from ammonia, which is produced by breaking apart hydrogen (H2) and nitrogen (N2) molecules and combining the component elements into ammonia gas (NH3) via the Haber-Bosch process. The process necessitates extremely high pressures, temperatures, and an iron catalyst.
The reaction’s exceptionally high pressure and temperatures—around 200 atm and 500 °C (932 °F)—require significant energy. Since ammonia is widely utilized in fertilizers and other sectors, global production requires a significant amount of energy.
To help minimize ammonia’s energy footprint, RIKEN CSRS researchers created a more environmentally friendly and energy-efficient reaction that can run stably at considerably lower temperatures without deactivating.
The most difficult challenge was breaking down nitrogen gas, which has a tight triple connection between its two nitrogen atoms.
The trick was to use ultrasmall molybdenum metal particles prepared from a hexanuclear molecular metal halide cluster, which was then activated with hydrogen gas.
Satoshi Kamiguchi, Senior Research Scientist, Advanced Catalysis Research Group, RIKEN Center for Sustainable Research Science
Once activated, numerous molybdenum atoms work together to swiftly break the strong nitrogen-nitrogen bonds, producing ammonia. When tested, this novel approach continuously produced ammonia from nitrogen and hydrogen gases for more than 500 hours at 200 °C (392 °F), significantly lowering the temperature necessary for the traditional Haber-Bosch process.
In addition to affecting the fertilizer industry, the new method of generating ammonia could indirectly help reduce carbon emissions if ammonia fuel is utilized globally. Ammonia fuel can be burned directly in internal combustion engines without producing CO2, but it has not become a viable alternative due to the high-energy Haber-Bosch process.
One advantage of the new technology is that it allows for lower-energy ammonia synthesis, which will significantly cut carbon emissions if ammonia fuel is employed on a large scale.
Ammonia stores nitrogen for fertilizer while also storing hydrogen. This makes it an excellent carrier for hydrogen, which some regard as the most efficient energy source. When stored hydrogen is required, it can be separated from ammonia and utilized as fuel without generating carbon dioxide.
Kamiguchi added, “Replacing the Haber-Bosch process with our new method should result in worldwide energy-saving. If ammonia fuel and hydrogen fuel are used in much larger amounts, vastly reducing the energy needed to synthesize ammonia will lead to lower CO2 emissions and help prevent further global-warming.”
One difficulty remains. The hydrogen required to generate ammonia is still produced using fossil fuels, and in sufficient quantities would result in massive CO2 emissions and energy consumption.
“When our catalyst system is combined with green H2 production from renewable energy, the emission of global-warming CO2 could be reduced even more,” Kamiguchi concluded.
The study team is working on adding promoters to the molybdenum-based catalyst, improving ammonia synthesis efficiency.
Journal Reference:
Kamiguchi, S., et al. (2024) Catalytic ammonia synthesis on HY-zeolite-supported angstrom-size molybdenum cluster. Chemical Science. doi:10.1039/D3SC05447K