In a recent paper published in the journal National Science Review, researchers from the University of Science and Technology of China proposed a novel strategy to immobilize and stabilize single-atom Cu sites within a metal-organic framework-based catalyst.
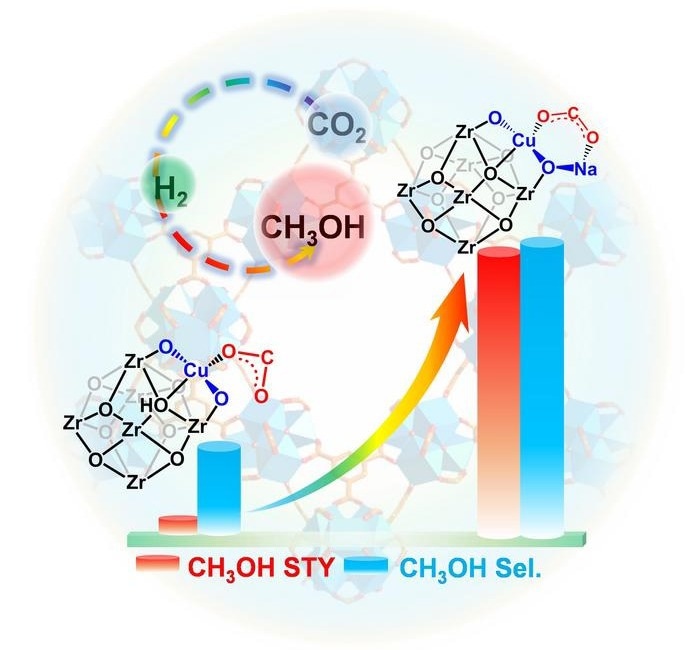
This image shows the performance of carbon dioxide hydrogenation using the Cu single atom catalysts without (left) or with (right) the presence of Na+-decorated microenvironment (reaction conditions: CO2/H2 volume ratio of 1/3 with reaction pressure of 3.5 MPa and reaction temperatures from 150 to 275 °C). The red columns represent the space-time yield of methanol, while the blue columns represent the selectivity of methanol. Notably, the introduction of Na+-decorated microenvironment has significantly enhanced the catalytic performance of the Cu single-atom catalysts. Image Credit: Science China Press
The buildup of carbon dioxide (CO2) in the Earth’s atmosphere since the start of the Industrial Revolution has given rise to serious environmental and climate-related concerns. In response to this urgent challenge, direct hydrogenation—the process of converting CO2 into chemicals and/or fuels—has become a widely accepted and essential tactic for reducing CO2 emissions and the use of fossil fuels.
Copper (Cu)-based catalysts have drawn the most attention among the various catalysts studied for CO2 hydrogenation. This is due to their promising potential in the methanol production process. The intrinsic reduction and aggregation tendencies of the Cu-based active centers, especially at elevated operating temperatures, pose a significant challenge to the practical application of Cu-based catalysts in CO2 hydrogenation despite the catalysts' promising catalytic activity.
This tendency toward reduction and aggregation may lead to larger Cu particles, which in turn may reduce the CO2 hydrogenation activity and produce unwanted CO byproducts. This makes it difficult to simultaneously achieve the high catalytic activity and methanol selectivity required for large-scale industrial applications.
A novel strategy to immobilize and stabilize single-atom Cu sites within a metal-organic framework-based catalyst has been proposed by the research team led by Professor Hai-Long Jiang from the University of Science and Technology of China (USTC) in response to these challenges.
The strategy involves creating a Na+ decorated microenvironment in close proximity to the single-atom Cu sites. Their extensive theoretical and experimental calculations have revealed the significance of the Na+-decorated microenvironment surrounding the single-atom Cu sites.
Through the electrostatic interaction between Na+ and Hδ- species, this microenvironment is essential in maintaining the atomic dispersion of Cu sites during the CO2 hydrogenation process, even at high temperatures up to 275 °C. The catalyst has superior CO2 hydrogenation activity (306 g·kgcat-1·h-1), high selectivity to methanol (93 %), and long-term stability compared to its counterpart without Na+ due to the remarkable stabilization effect of single-atom Cu sites.
In addition to advancing the development of Cu-based catalysts for the selective hydrogenation of CO2 to methanol, this ground-breaking work presents a practical method for forming stable single-atom sites in advanced catalysis through the creation of nearby alkali-decorated microenvironments.
Journal Reference:
Guan, X., et al. (2024) Promoted hydrogenation of CO2 to methanol over single-atom Cu sites with Na+ decorated microenvironment. National Science Review. doi.org/10.1093/nsr/nwae114